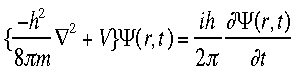
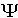 is the wavefunction, m is the mass of the particle, his Plancks constant, V is the potential field in which the particle is moving
is the wavefunction, m is the mass of the particle, his Plancks constant, V is the potential field in which the particle is moving
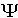 would be a function of the coordinates of all the particles in the system as well as time t.
would be a function of the coordinates of all the particles in the system as well as time t.
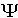 subject to the appropriate boundary conditions. Many different wavefunctions are solutions to it, corresponding to different stationary states.
subject to the appropriate boundary conditions. Many different wavefunctions are solutions to it, corresponding to different stationary states.