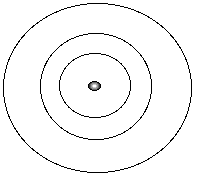
Bohr Atom:
| Electrons that are "particles" that revolve around the nucleus in orbits. These orbits are at fixed distances from the nucleus. Electrons can move between orbits, using or releasing energy in the process. |  |

where h = Planck's constant
n = quantum number (orbit number)
