Schrodinger's Equation
in this equation, the complex conjugate of the wavefunction on psi ( ) is interpreted as the probability density of the particle.
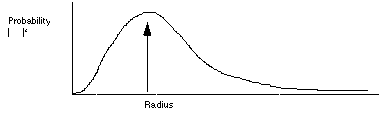 the Schrodinger equation for a collection of particles (a molecule) is similar
the Schrodinger equation for a collection of particles (a molecule) is similar
- Psi is a function of the coordinates of all the particles in the system as well as time
- the energy and many other properties of the particle can be obtained by solving the equation
- multiple solutions are the rule, corresponding to different stationary states
Home
Contents
Previous
Next
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
22 |
23 |
24 |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
33 |
34 |
35 |
36 |
37 |
38 |



