Electron Configurations
Electron configurations are a method of accounting for where electrons are, as well as the shape of electron clouds and certain properties of an element. These observed properties are also responsible for the shape of the periodic table. In this system, any electron has four quantum numbers, which are unique to that electron in that atom. These quantum numbers are used to specify different wavefunctions which represent the electron.
- n
This is known as the principle quantum number, which denotes the energy level. This is similar to the "orbits" in the Bohr model, now called "shells". Shell size increases with n.
- l
Called the secondary quantum number, l can have a value from 0 to n-1. Every value of l stands for a differently shaped sub-shell. These are named s,p,d,f... for increasing values of l.
- m
The magnetic quantum number separates the sub-shells into individual orbitals. It has values of -l to +l. For example, an s sub-shell (l=0) has only one orbital, while a p sub-shell (l=1) has three orbitals. Every orbital has a different shape or orientation. Sometimes this orientation is described in terms of x,y,z coordinates instead of m.
- ms
Spin quantum number, with a value of +1/2 or -1/2, differentiates between two electrons in a pair which share an orbital. Also given a value of "up" or "down".
Orbitals are named according to the shell, letter of the sub-shell, and orientation of the orbital. Most orbitals have descriptive (x,y,z) designations of their orientation, but we are not concerned with those.
- Examples:
- hydrogen has a 1s1 configuration: 1 electron in an "s" orbital at n=1
- carbon has a 1s2 s2s 2p2 configuration for a total of six electrons
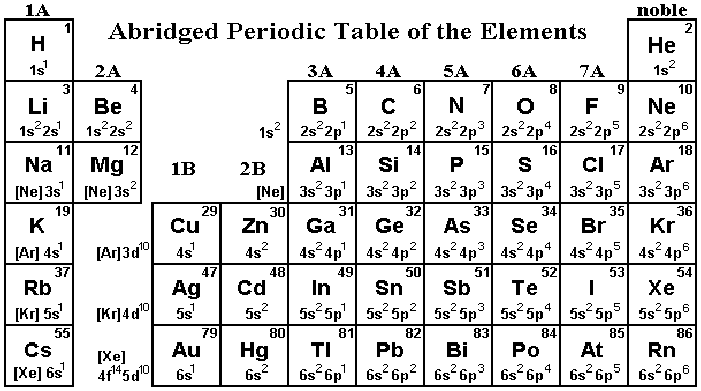
- Periodic Table shows basic electron configurations
We have the application Atom in a Box available for you to use. This allows you to examine the three dimensional shapes of electron clouds as based on wavefunctions. See the Quick Guide to Atom in a Box for more information!
[Previous][Next]
[CompChem Main Page][Contents]
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[25]
[26]
[27]
[28]
[29]
[30]
[31]
[32]
[33]
[34]
Developed by
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.
Copyright © 1998
Last Modified:
Questions or comments about this page should be directed to gotwals@shodor.org
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.