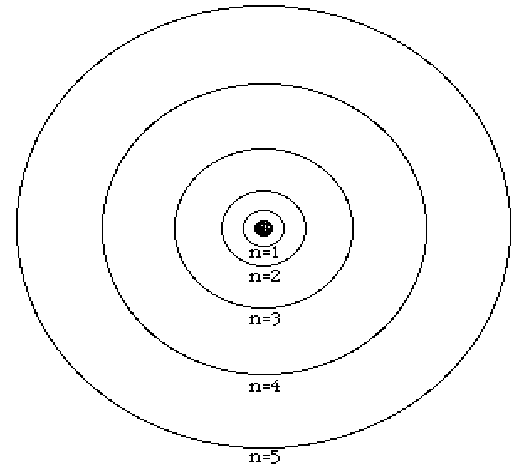

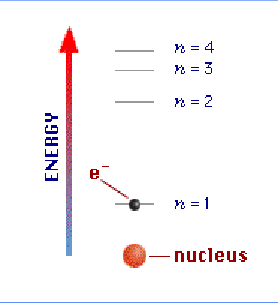
- as the electron moves "up" to the next "n" level, the energy increases
- electrons prefer to be in the lowest possible level, called the "ground state"
- when electrons fall from a high level to a low level, energy is released, in the form of light
 |  |
 |
|
[CompChem Main Page][Contents]
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[25]
[26]
[27]
[28]
[29]
[30]
[31]
[32]
[33]
[34]
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.