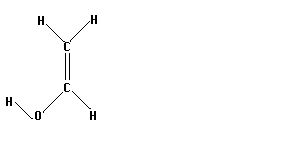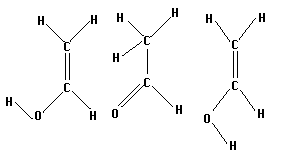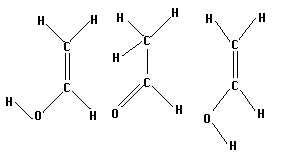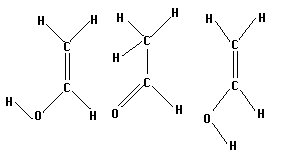
Vinyl alcohol, or acetaldehyde, has several isomers which can be isolated. Three of these are shown below. The two on the right are the keto and enol forms of acetaldehyde, respectively. The left most molecule is simply the enol form with the furthermost hydrogen at a different angle.

Construct all three isomers and optimize them. Then select two isomers, search for a transition state, and save it. Run a frequency calculation as usual to test your results.
How are the transition states that you find similar or different? Is it possible to find a transition state from the leftmost isomer to the keto form without rotating the hydrogen (ie, going through the enol form)? If so, how does the energy of this form compare to the energy of the transitions through the keto configuration?
To Slides:[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[25]
[26]
[27]
[28]
[29]
[30]
[31]
[32]
[33]
[34]
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.