Transition Structure Searches
Transition structures are also called "intermediary products". As this name implies, they are a "step" along the way between reactant and product. Transition states are inherently unstable, since they are high-energy geometries. The energy of the transition state is also called the "energy of activation" or "activation energy", since it is the energy required for the reaction to proceed.
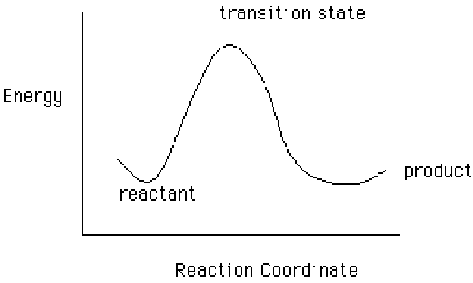
Transition State calculations are useful for
- Finding the activation energy
- Determining a mechanism for a reaction (how it physically happens)
- Understanding the difference between several potential mechanisms
- Generating reaction energy graphics (like the one above)
Calculating transition states is a multi-step process
- build one of the reactants and one of the products (these must be isomers)
- modify the product to represent the transition state geometry (this is an intuitive guess)
- request a transition search
- pair up the corresponding atoms in reactant and product
- generate and save the transition state search
- perform a transition search, then calculate the frequencies
- ONE AND ONLY ONE imaginary frequency means you have a possible transition state
[Previous][Start]
[CompChem Main Page][Contents]
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[25]
[26]
[27]
[28]
[29]
[30]
[31]
[32]
[33]
[34]
Developed by
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.
Copyright © 1998
Last Modified:
Questions or comments about this page should be directed to gotwals@shodor.org


 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.