Quantum Theory in Chemistry
Quantum Model:
Electrons are not particles, but have wavelike charactics. Schrodinger's equation:
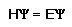
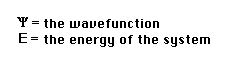
where H =a nasty mathematical operator (the "Hamiltonian")
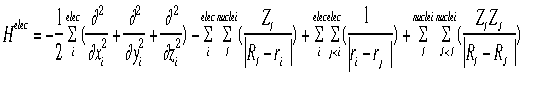
is used to calculate the probability of an electron being at a certain place.
Schroedinger's equation is called an eigenfunction, which means that if an operation is performed on it, the result is the same function (perhaps times a constant)
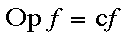
[CompChem Main Page][Contents]
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[25]
[26]
[27]
[28]
[29]
[30]
[31]
[32]
[33]
[34]
Developed by
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.Copyright © 1998
Last Modified:
Questions or comments about this page should be directed to gotwals@shodor.org