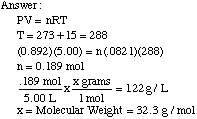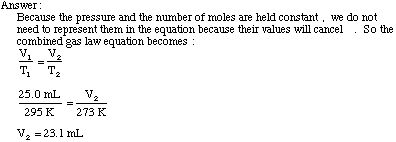Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Gas LawsGases behave differently from the other two commonly studied states of matter, solids and liquids, so we have different methods for treating and understanding how gases behave under certain conditions. Gases, unlike solids and liquids, have neither fixed volume nor shape. They are molded entirely by the container in which they are held. We have three variables by which we measure gases: pressure, volume, and temperature. Pressure is measured as force per area. The standard SI unit for pressure is the pascal (Pa). However atmospheres (atm) and several other units are commonly used. The table below shows the conversions between these units.
Volume is related between all gases by Avogadro's hypothesis, which states: Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. From this, we derive the molar volume of a gas (volume/moles of gas). This value, at 1 atm, and 0° C is shown below.
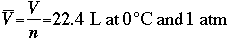
Where:
V bar=molar volume, in liters, the volume that one mole of gas occupies
under those conditions An equation that chemists call the Ideal Gas Law, shown below, relates the volume, temperature, and pressure of a gas, considering the amount of gas present.
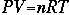
Where:
P=pressure in atm
The Ideal Gas Law assumes several factors about the molecules of gas. The volume of the molecules is considered negligible compared to the volume of the container in which they are held. We also assume that gas molecules move randomly, and collide in completely elastic collisions. Attractive and repulsive forces between the molecules are therefore considered negligible. Example Problem: A gas exerts a pressure of 0.892 atm in a 5.00 L container at 15 degrees Celsius. The density of the gas is 1.22 g/L. What is the molecular weight of the gas?
We can also use the Ideal Gas Law to quantitatively determine how changing the pressure, temperature, volume, and number of moles of substance affects the system. Because the gas constant, R, is the same for all gases in any situation, if you solve for R in the Ideal Gas Law and then set two Gas Laws equal to one another, you have the Combined Gas Law:
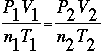
Where: values with a subscript of "1" refer to initial conditions
If you know the initial conditions of a system and want to determine the new pressure after you increase the volume while keeping the numbers of moles and the temperature the same, plug in all of the values you know and then simply solve for the unknown value.
Example Problem: A 25.0 mL sample of gas is enclosed in a flask at 22
degrees Celsius. If the flask was placed in an ice bath at 0 degrees
Celsius, what would the new gas volume be if the pressure is held constant? We can apply the Ideal Gas Law to solve several problems. Thus far, we have considered only gases of one substance, pure gases. We also understand what happens when several substances are mixed in one container. According to Dalton's law of partial pressures, we know that the total pressure exerted on a container by several different gases, is equal to the sum of the pressures exerted on the container by each gas.
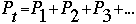
Where: Pt=total pressure in atm
Using the Ideal Gas Law, and comparing the pressure of one gas to the total pressure, we solve for the mole fraction.
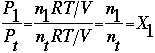
Where: X1=mole fraction of gas "1" And discover that the partial pressure of each the gas in the mixture is equal to the total pressure multiplied by the mole fraction.
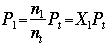
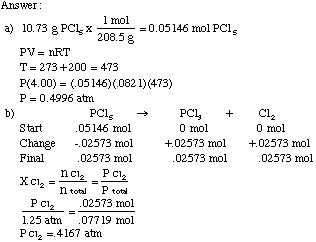
Example Problem: A 10.73 g sample of PCl5 is placed in a 4.00
L flask at 200 degrees Celsius.
As we stated earlier, the shape of a gas is determined entirely by the container in which the gas is held. Sometimes, however, the container may have small holes, or leaks. Molecules will flow out of these leaks, in a process called effusion. Because massive molecules travel slower than lighter molecules, the rate of effusion is specific to each particular gas. We use Graham's law to represent the relationship between rates of effusion for two different molecules. This relationship is equal to the square-root of the inverse of the molecular masses of the two substances.
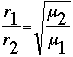
Where: r1=rate of effusion in molecules per unit time of gas "1"
Previously, we considered only ideal gases, those that fit the assumptions of the ideal gas law. Gases, however, are never perfectly in the ideal state. All atoms of every gas have mass and volume. When pressure is low and temperature is low, gases behave similarly to gases in the ideal state. When pressure and temperature increase, gases deviate farther from the ideal state. We have to assume new standards, and consider new variables to account for these changes. A common equation used to better represent a gas that is not near ideal conditions is the van der Waals equation, seen below.
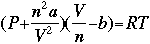
Where the van der Waals constants are: a accounts for molecular attraction
The table below shows values for a and b of several different compounds and elements.
Practice Ideal Gas Law Problem: 2.00 g of hydrogen gas and 19.2 g of oxygen gas are placed in a 100.0 L container. These gases react to form H2O(g). The temperature is 38 degrees Celsius at the end of the reaction. a) What is the pressure at the conclusion of the reaction? b) If the temperature was raised to 77 degrees Celsius, what would the new pressure be in the same container? Ideal gas law solution.
Practice Pressure Problem: a) If the total pressure in the container is 5.00 atm, what are the partial pressures for the three gases remaining? b) Using Graham's Law, what is the ratio of the effusion rates of NH3(g) to O2(g)? Pressure solution.
Compressibility and Ideal Gas Approximations: An Online Interactive Tool
[Advanced Index ]
[Gas Laws]
[Thermodynamics]
[Kinetics]
[Equilibria] | ||||||||||||||||||||||||||||
 The Shodor Education Foundation, Inc.
The Shodor Education Foundation, Inc.in cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1998
Last Update:
Please direct questions and comments about this page to
WebMaster@shodor.org