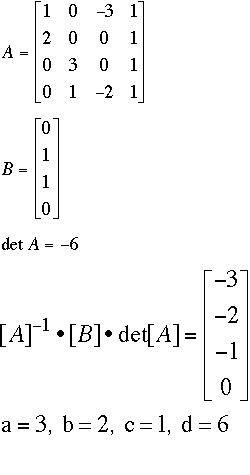The trick with this one is to treat the phosphate ion (PO4-3) as an element, and not break it into two individual elements.If you do that, you get:
Fe: 1a + 0b -3c = 0d
Cl: 2a + 0b + 0c = 1d
Na: 0a + 3b + 0c = 1d
(PO4): 0a + 1b - 2c = 0d
Note that you do not have a square matrix for Matrix A. Following the regular procedures, you should get a 4 x 3 matrix (four rows, three columns). You can make it square without changing the algorithm by adding a column of 1's at the end of the matrix.