|
|
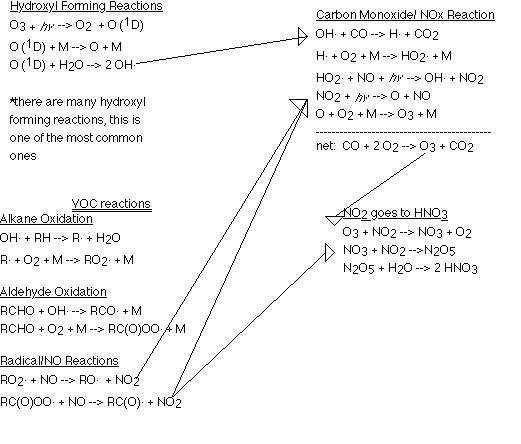
Formation of Photochemical SmogAs we know, photochemical smog is composed primarily of ozone, NOx, and PAN. These species all interact with each other, for example, ozone comes about as a result of the reaction between NOx and VOC. The following schematic shows the relationship between the various species in smog. When looking at the schematic, take special note of the important role that radicals play in the different reactions.

At the bottom of the image, NO and hydrocarbons are released from burning fossil fuels. NO reacts with O3 or RO2
 Now, lets try a runnable model for photochemical smog formation: First, read the Background to the model Next, go to the Runnable Model!!
Confused? Have a question? If so, check out the Frequently Asked Questions (FAQ) page or send mail to the OS411 tutor (os411tutor@shodor.org) with your question! Report technical/content problems here |
|
|