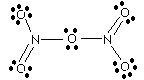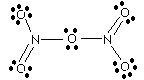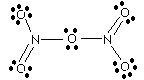
Shodor Homepage
Activity Materials
Introduction
About this resource
Educational Objectives
Mathematics
Building the Model
Run the Model
Glossary of Terms
Credits
Related Links
Contact Webmaster
|
Case Study: Nitrogen Pentoxide Kinetics
In this case study, you are asked to make qualitative observations about the effect of temperature on the decomposition of nitrogen pentoxide. You are also asked to investigate the question: does the time required for a given fraction of a reactant to decompose via a first order reaction depend on the concentration? In other words, how much time does it take for half of a starting concentration of N2O5 to decompose?
The reaction mechanism is:
2 N2O5 -----> 4 NO2 + O2
This is a first-order kinetic reaction, based on:
rate = k[N2O5]
|