Application: Smog photochemistry in the troposphere
Photochemical smog is an atmospheric condition that produces severe eye irritation
and poor visibility, to name just two of the effects. Three ingredients -- energy
from a light source (ultraviolet), hydrocarbons, and nitrogen oxides -- are needed
for photochemical smog to be formed. Two of those components are produced through the burning
of fossil fuels, most notably automobiles. Photochemical smog is also sometimes
known as "oxidizing smog", in that it has a high concentration of oxidizing agents.
Ozone is a common oxidizing agent found in photochemical smog. Another type of smog, "reducing smog", has high concentrations of sulfur dioxide, which is a reducing agent. Its presence has historical significance in studies done in places like London,
which used sulfur-containing coal as its main energy source.
A schematic is again useful in understanding the chemistry of smog production in the
troposphere:
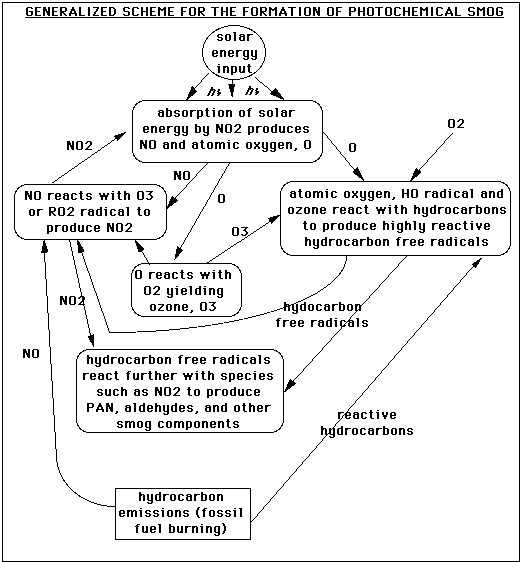
This graphic is relatively self-explanatory. It begins at the bottom with the production
of NO and reactive hydrocarbons by fossil fuel burning (such as an automobile).
On the left side, the NO reacts with tropospheric ozone or a hydrocarbon radical
(RO2 ) to produce NO2 (a radical is a molecule fragment that has an unpaired electron). This absorbs solar energy (represented by the letters hv) to create NO (which propagates the system) and atomic oxygen. Atomic oxygen reacts to form tropospheric ozone, which feeds back into the NOx system (the "x" here refers
to the number of oxygens, and serves as a general notational term for the nitrogen oxides). Atomic oxygen can also react with hydroxyl radicals, OH , and ozone to
form the reactive hydrocarbon radicals utilized in the NOx system. These radicals
also react to form other components of smog, such as PAN (peroxyacetyl nitrate) and
aldehydes (RC=OH, where R is some hydrocarbon chain). , and ozone to
form the reactive hydrocarbon radicals utilized in the NOx system. These radicals
also react to form other components of smog, such as PAN (peroxyacetyl nitrate) and
aldehydes (RC=OH, where R is some hydrocarbon chain).
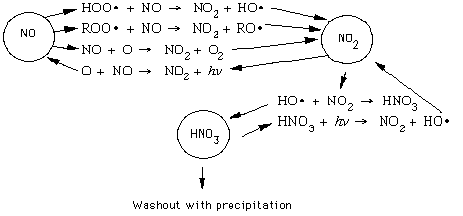
The graphic below shows the essential workings of the NOx system, with the interactions
between NO and NO2 on the left and the production/washout of HNO3 on the right:
We can also look at the formation of photochemical smog from a kinetic perspective.
This chart shows the nine key equations of smog production, and the rate constant
that affects the speed (or rate) at which the reaction takes place:
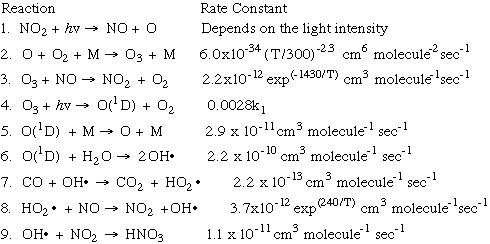
In this graphic, we see the involvement of the NOx system and the production of ozone.
Here is a narrative version of the graphic above:
- Reaction 1: NO2, reacts with light energy, hv
, to form NO and a singlet oxygen atom. The rate of this reaction
depends on how much light energy there is -- a sunny day versus a cloudy day!
- Reaction 2: singlet oxygen reacts with the oxygen molecule (what you breathe) in
the presence of a catalyst "M" to form ozone, O3. The catalyst M remains unchanged (which is the definition of a catalyst!). The
rate of this reaction depends on the temperature in the atmosphere.
- Reaction 3: Ozone reacts with NO to produce more NO2 and O2. These products feed back into Reactions 1 and 2, thus ensuring a steady production
of ozone! The rate of this reaction also depends on the temperature in the atmosphere.
- Reaction 4: Ozone is degraded (broken down) by light energy, forming a charged
form of singlet oxygen, O(1D), and more molecular oxygen. Notice that this reaction proceeds at a much slower
rate than the first reaction (about one-fourth of one percent as slow!)
- Reaction 5: the charged oxygen reacts with a catalyst to return to its normal state
of singlet oxygen (which is, by the way, poisonous to breathe!)
- Reaction 6: some of the charged oxygen reacts with water in the atmosphere to form
a hydroxyl radical, OH
 . Radicals are fragments of molecules that have at least
one unpaired electron, and are highly reactive. The hydroxyl radical, for example,
is responsible for the majority of the chemical reactions that happen in the atmosphere
during the day. Other radicals take control at night-time when there is no energy
from the sun. . Radicals are fragments of molecules that have at least
one unpaired electron, and are highly reactive. The hydroxyl radical, for example,
is responsible for the majority of the chemical reactions that happen in the atmosphere
during the day. Other radicals take control at night-time when there is no energy
from the sun.
- Reaction 7: carbon monoxide in the atmosphere, produced by fossil-fuel burning
such as automobiles, reacts strongly with hydroxyl radicals to form carbon dioxide
and HO2
 radicals. radicals.
- Reaction 8: the HO2
 radicals formed in Reaction 7 react with the extra NO in the atmosphere to form
more NO2 and more OH radicals formed in Reaction 7 react with the extra NO in the atmosphere to form
more NO2 and more OH radicals. The rate of this reaction is dependent on the temperature
in the atmosphere. radicals. The rate of this reaction is dependent on the temperature
in the atmosphere.
- Reaction 9: the hydroxyl radicals react with NO2 to form nitric acid, HNO3, which will eventually be one of the culprits in the formation of acid rain, but that's (thankfully!) another model!
In summary: the development of photochemical smog is dependent upon solar radiation, source emissions
of hydrocarbons and nitrogen oxides, and atmospheric stability (for enhanced concentrations).
Early in the morning, commuter traffic releases NO and hydrocarbons. At the same time, NO2 may decrease through because the sunlight can break it down to NO and O. The O is then free to react with O2 to form O3. Shortly thereafter, oxidized hydrocarbons react with NO to increase NO2 by midmorning. This reaction causes NO to decrease and O3 to build up, producing a midday peak in O3 and minimum in NO. As the smog ripens, visibility may be reduced due to light scattering by aerosols. Primarily due to the dependence on commuter traffic between surburbs and cities, there
are presently more than 40 urban areas in violation of the US ambient air quality standard for ozone.
|

