Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
The Arrhenius EquationBackground reading:Common sense and chemical intuition suggest that the higher the temperature, the faster a given chemical reaction will proceed. Quantitatively this relationship between the rate a reaction proceeds and its temperature is determined by the Arrhenius Equation. At higher temperatures, the probability that two molecules will collide is higher. This higher collision rate results in a higher kinetic energy, which has an effect on the activation energy of the reaction. The activation energy is the amount of energy required to ensure that a reaction happens.This calculator calculates the effect of temperature on reaction rates using the Arrhenius equation.
R has the value of 8.314 x 10-3 kJ mol-1K-1 You should use this calculator to investigate the influence of temperature on the rate coefficient. This calculator allows you to perform three different calculations:
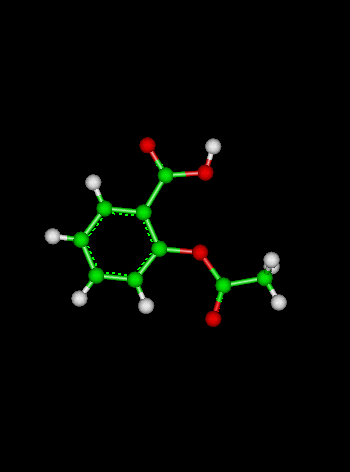
Sample Problem:The reaction:
has a rate coefficient of 1.0 x 10-10 s-1 at 300 K and an activation energy of 111 kJ mol-1. What is the rate coefficient at 273 K? (Solution: calculate the value of A for a temperature of 300 K, then use the calculated value of A to calculate k at a temperature of 273 K. We generally assume that A and the activation energy Ea do not vary with temperature).
|