Vibrational Frequencies of Ethylene
Objective: Students will learn about forensic analysis through calculating frequencies of molecule vibration.
Materials: Each student will need
- a computer with Mac/PCSpartan
- a sample vibrations worksheet
Overview
Frequency calculations are useful tools in computational chemistry for getting at the nature of a chemical structure. Molecules vibrate when they absorb light in the infrared region of the electromagnetic spectrum. The frequency at which light is absorbed as well as the intensity at which light is absorbed is a function of the geometry of the molecule. These features are, in fact, highly sensitive to minute changes in structure. Vibrational spectroscopy, which studies these relationships, is thus a major methodology used to investigate structures. If you recall your analytical chem days, you might have remember looking at infrared spectra. If you suspected a carbonyl (C=O) functional group, you would have looked for a peak around the 1675-1880/cm range. You might also recall looking at the region below 1500/cm, which is known as the fingerprint region of the spectrum. By comparison of peaks on the spectra to spectra of standard molecules, you were able to identify unknown samples.
Some basic observations about molecules:
- all molecules vibrate
- vibrations occur at specific frequencies, depending on the molecule/ functional group
- measurement of the frequencies of a molecule allows chemists to identify the molecule by a spectra, such as an infrared (IR) spectra
-
molecules have a specific number and type of vibrations, depending on "N", the number of
atoms
- number: 3*N-6 for nonlinear molecules, 3*N-5 for linear molecules
Types of Vibrations
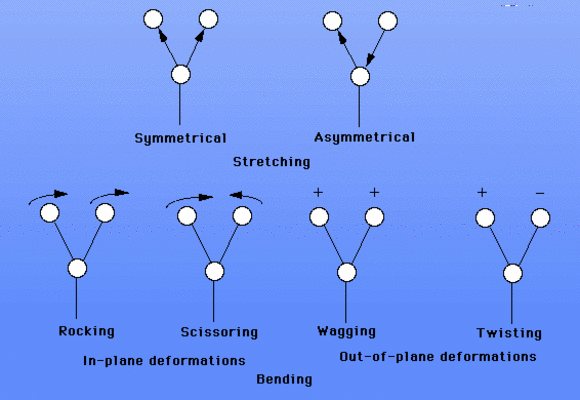
There are six different kinds of vibrational "motions" that we can observe:
-
Stretching vibrations
- symmetrical
- asymmetrical
-
Bending vibrations
-
In-plane vibrations
- rocking
- scissoring
-
Out-of-plane vibrations
- wagging
- twisting
-
In-plane vibrations
Graphically, the vibrations look like this:
Directions
Construct the ethylene (H 2 C=CH 2 ) molecule on Mac/PCSpartan. Perform an ab initio calculation on the molecule. The choice of level is up to you; think about (and if needed, discuss) what level you should use, based on your knowledge of different theories, calculation time, and purpose of the calculation. Make sure that you perform a geometry optimization! Otherwise, your frequency calculations will be meaningless. In your "setup" procedure, make sure that you click on the "Frequencies" under the "Compute" menu.
Once you have submitted your job, choose "Vibrations" from the display menu. You can view each of the vibrations by clicking on the vibrations. You can increase the size and speed of the vibrations with the various options.
The goal of this project is to learn to recognize the six different vibrations in chemistry. Use your worksheet on the sample vibrations for the ethylene molecule to identify each vibrational frequency. Submit your report identifying each of the vibrations with its vibrational frequency. The arrows on the worksheet represent "in-plane" vibrations, and the plus (+) and minus (-) symbols represent "out-of-plane" vibrations. You should rotate your molecule to allow you to visualize the various vibrations.