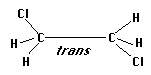 | 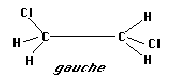 |
Dichloroethane has two forms which we will deal with in this lab, trans and gauche. The gauche form appears on first examination to have a much higher energy because of the larger dipole moment. However, in this study you will examine the energy difference between these two molecules singly and in solution using semi-empirical calculations. A difference of approximately 1 kcal/mol makes the more stable configuration appear ten times more often than the less stable geometry. So, even a difference of that size can have an appreciable effect on which geometry is more abundant.
Construct both forms of dichloroethane, as shown below.
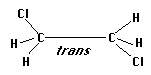 | 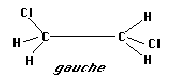 |
Optimize the geometry of each form using both AM1 and AM1-SM2 theory (this means you should do *four* optimizations!) What is the energy difference between the two molecules in solution? in gas phase? In which phase is the gauche configuration more common? Compare the dipole moment of the two molecules. Compare and discuss your results with your classmates. What can you conclude about the effect of polar solvents on polar and nonpolar solute?
To Slides:[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[25]
[26]
[27]
[28]
[29]
[30]
[31]
[32]
[33]
[34]
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.