In this study, you will investigate the correlation between electrostatic potential and pKa for a group of structurally similar acids.
pKa is a measure of the strength of an acid. The corresponding measure for bases is pKb. For both of these constants, a higher numerical value means a weaker acid or base. As you will see below, these are moderately strong acids.
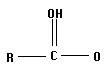
The acids you will investigate, called carboxylic acids, all have a carboxyl group on one end. A carboxyl group is also written as COOH, with a C double bonded to an oxygen and bonded to an oxygen with a hydrogen on the other side, as in the structure below. That hydrogen is the center of the electrostatic potential which we will study. The "R" on the structure below can be any atom or group of atoms.

Construct and optimize the acids shown below. Be sure to include an electron density surface with electrostatic potential (elpot) as a property in the calculation, and an electrostatic potential surface if you wish.
| Structure of Acid | Experimental pKa | Machine responsible |
1. 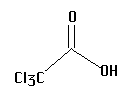 | 0.70 | pea |
2. 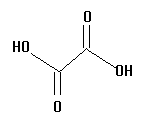 | 1.23 | surgeon |
3. 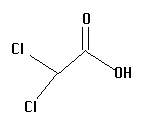 | 1.48 | surgeon |
4. 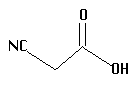 | 2.45 | mid |
5. 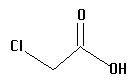 | 2.85 | mid |
6. 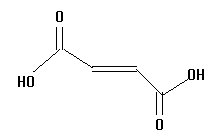 | 3.10 | pea |
7. 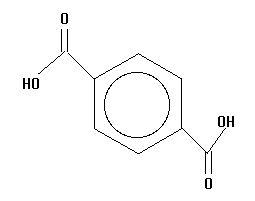 | 3.51 | damage |
8. 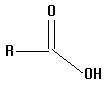 | 3.75 | child |
9. | 3.79 | child |
10. 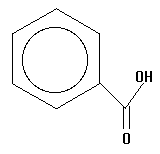 | 4.19 | fore |
11. 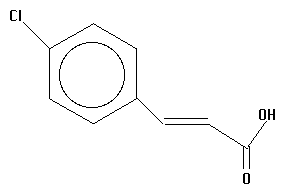 | UNKNOWN | UNKNOWN |
12. 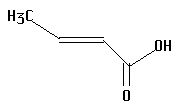 | 4.70 | fore |
13. 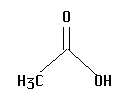 | 4.75 | no |
14. 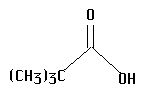 | 5.03 | no |
Measure and record the electrostatic potential by the end hydrogen. Rembember, this hydrogen is the one attached to an oxygen which is attached to a carbon! Plot potential against pKa and interpret the results. Is there a linear correlation? What is the slope (sign and numeric value)? Can potential be used to predict pKa and vice versa?
Recalculate the electrostatic potentials for these acids in solution. How does this change the correlation you observed earlier?
[Glossary][CompChem Main Page][HTML Labs]
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.