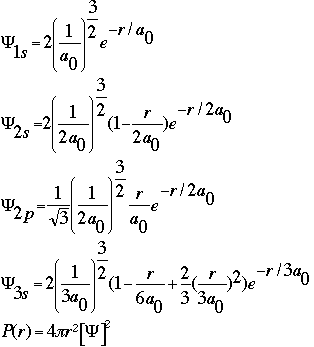
We want to evaluate the probabiltiy of finding ONE electron at three different levels (orbitals) in a hydrogen atom.
The wavefunction is the basic algorithm that we will use for this, but it does not directly represent probability. As you see in the algorithm, the wavefunction squared times the radius is an actual probability distribution. The wavefunction depends on radius, measured in picometers (pm) and a constant, the Bohr radius. The Bohr radius is written as a0 and is the distance of the nucleus to the first orbit in the Bohr atom. Its value is 52.9pm. Below are the algorithms needed.
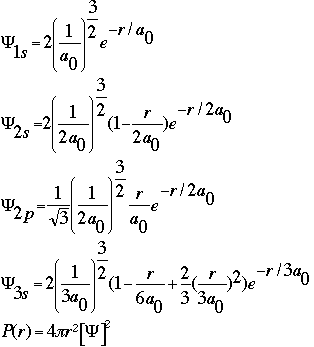
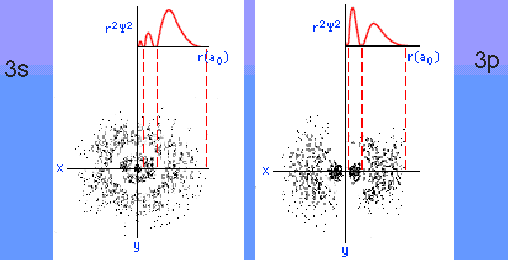
Think about, then discuss with your classmates, what you think these probability distributions should look like. Recall what you know about the shape of electron clouds.
Create a spreadsheet which contains the following elements:
Work smarter, not harder... use the fill function, etc. to make this as painless as possible!
Below is a sample of the spreadsheet. Hint: you need to investigate many more values of r than are shown here!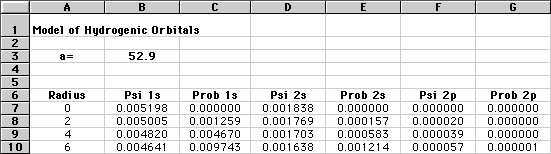
Discuss and compare your results with those of your classmates. What do these probabilities mean in three dimensional space?
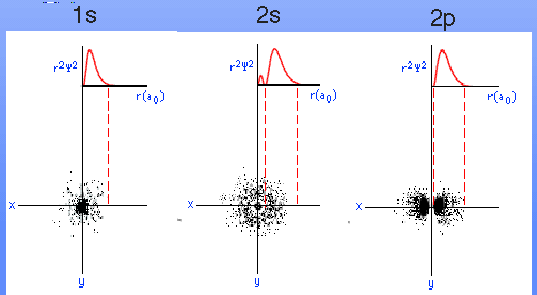
Below is a set of graphical interpretations which may help you compare your probability distributions to three-d electron clouds.
 |
 |
[Glossary][CompChem Main Page][HTML Labs]
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.