LUMO of Planar and Perpendicular Benzyl Cation
Background
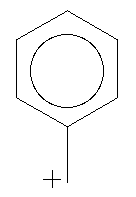
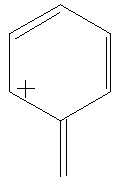
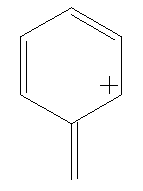
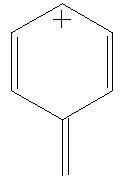
Benzyl cation consists of a benzene ring with an extra carbon bond (which may be a single or double bond, depending on the resonance structure being used) added in place of a hydrogen. This has a postive charge. The charge is traditionally considered to reside along the carbons next to and directly opposite the bond (this is represented in the four resonance structures).
In this study you will investigate the relationship between this postive charge and the lowest unoccupied molecular orbital, or LUMO for two different alignments of benzyl cation.
Case Study
Construct benzyl cation in the planar alignment for one of the resonance structures, with the hydrogens of the added carbon in a plane with the benzene ring. Optimize its geometry and calculate a LUMO surface. (Make sure that you tell MacSpartan that you are working with an ion: put the charge in the appropiate box before conducting the calculations!) Where is the LUMO concentrated? How does this compare with the location of the postively charged parts of the molecule?
Construct the perpendicular alignment of the same resonance structure, with the hydrogens of the added carbon perpendicular to the plane of the benzene ring. Optimize the geometry and calculate a LUMO surface. Compare this to the earlier configuration and the positive charge on the molecule. What do you think LUMO represents, in terms of charge? What does it represent in terms of reactions which the molecule can undergo? Compare and discuss your results with your classmates.
Extensions
Calculate the LUMO surfaces, in both alignments, for all of the resonance structures shown below. Do the different resonance structures exhibit different localizations of the positive charge? Can you correlate this with a traditional structure drawing?
[Glossary][CompChem Main Page][HTML Labs]
Developed by
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.
Copyright © 1998
Last Modified:
Questions or comments about this page should be directed to gotwals@shodor.org




 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.