

Energy is stored in the covalent bonds between each phosphate group which make up the tail of the molecule. The last phosphate bond holds the most energy (approx. 7 kcal/mole). It is called the pyrophosphate bond. In order to release it's energy to the body, ATP breaks down into ADP [Adenosine Diphosphate(2 phosphates)] and an inorganic phosphate group and releases energy from the pyrophosphate bond.
ADP is an exergonic molecule which means that it yield energy when formed. When ADP reacts comes in contact with enough energy and an inorganic phosphate (PO4) it becomes ATP and stores energy yet again. To once again become ATP, ADP gets energy and its third phosphate from respiration. More ATP forms from aerobic respiration (respiration where air is present) than from anaerobic respiration (respiration where air is absent) because there is more energy involved. (For those who don't know, respiration is the process by which the body uses oxygen and food to provide energy for the body.) Anaerobic respiration provides the body with approx. 2 ATP molecules opposed to aerobic respiration's 38 ATP molecules. As you can see, aerobic respiration is more efficient.
The transition between ATP and ADP is constantly happening in the body as our body constantly needs energy to carry out the various bodily functions that sustains life.
ATP= ADP+inorganic phosphate+energy
ADP+inorganic phosphate+energy= ATP
Throughout the experiment, we chose to use AM1 as our theory for our basis set. Basically, we did this because it reduced our amount of CPU time. A semi-empirical method, such as AM1, was more computationally economical than using an ab initio method. An ab initio method, such as the 6-31G* basis set level of theory, would have been more desirable because its start-from-math-only approach produces greater accuracy, but a semi-empirical method was more computationally efficient than an ab intio method.
First, we built the portion of an ATP molecule that contained the site of the terminal bond. We did not build a model of an entire ATP molecule because it would have been too complex and too computationally expensive to do in the time allotted. Next, we built a model of ADP by breaking the terminal bond between the last two phosphate groups which can be done by constraining the bond length between the oxygen atom and the last phosphate group.
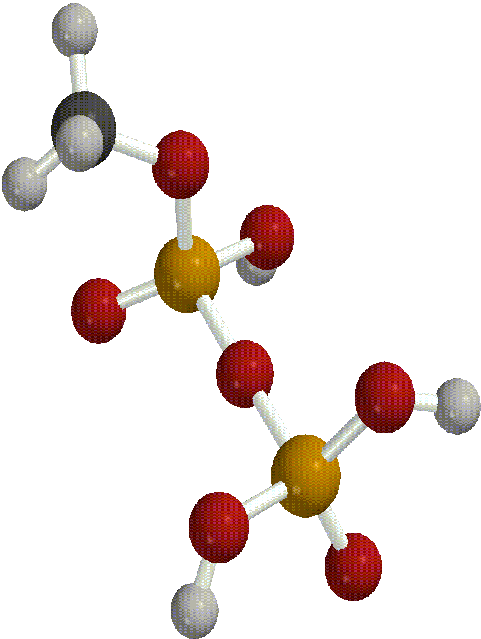
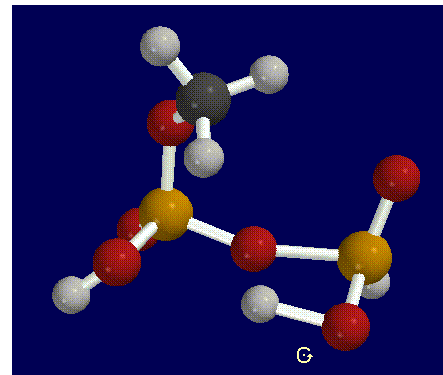
After building and geometry optimizing these molecules with a semi-empirical AM1 basis set theory, we requested a transition search in order to find the transition state of the reaction. In order to determine whether a transitional state was found or calculated for the reaction, you must run a frequency scan on the molecule produced by the transition search.
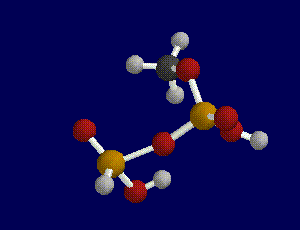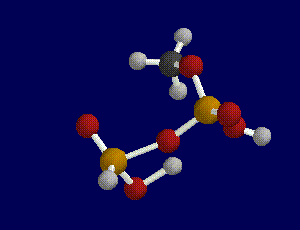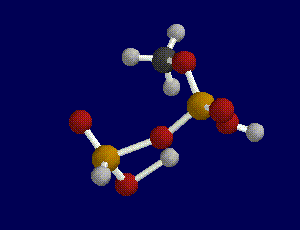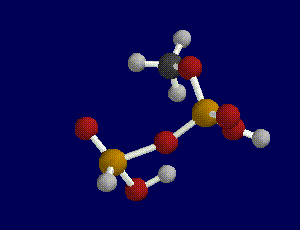
A transition state is indicated when one and only one imaginary frequency is found as a result of a frequency scan.
After determining that we had one and only one imaginary frequency and therefore had found the transition state of our portion of an ATP molecule, we initiated single point energy scans in each molecule.
Single point energy scans are used to predict the energy and related properties of a molecule during a reaction. Regularly, as the reactants of a chemical reaction combine, their energy levels tend to make a dramatic increase because they have to have enough energy to cross the energy barrier. The difference in the energy levels of the transition state and the reactants is called the activation energy. When we charted and graphed our results from the three molecular single point energy (SPE) scans, we discovered that our reaction had no energy barrier to cross and therefore, no activation energy or catalyst.
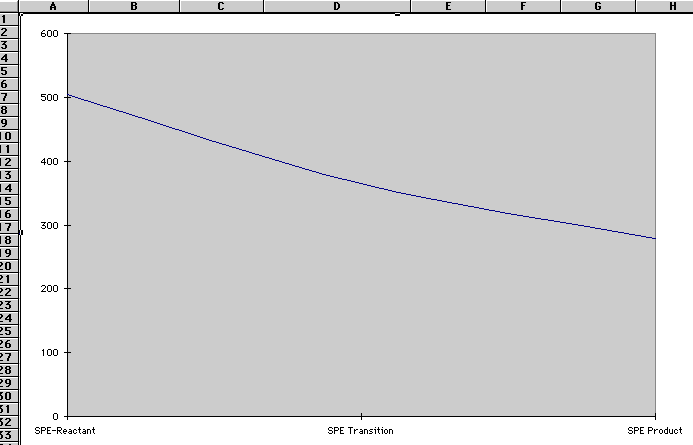
Graham, Kieth. Hicks, Laurel. Shimmen, Dolores. Thomson, George. Biology : God's Living Creation. A Beka Book Publications. (1986):137, 139, 141, 189, 221, 567, 576.
Hendrickson, James B., Cram, Donald J., Hammond, George S. Organic Chemistry. McGraw-Hill Book Company. (1970): 1040,1042,1043,1045,1047,1048.
Loundon, Marc G. Organic Chemistry, Second Edition. The Benjamin/Cummings Publishing Company, Inc.(1988): 1237, 1238.
Personal Communication: Gotwals, Robert, personal instruction, Aug. 18 - 22, 1997 Thissen, Anne, personal instruction, Aug. 18 - 22, 1997 Web pages BIO 181: General Biology (Majors) I: Respiration. Pima Community College, Tucson, AZ. http://west.cscwc.pima.edu/~achristensen/respiration.html
M.J. Farabee. ATP and Biological Energy. http://www.emc.maricopa.edu/Bio/BIO181/bio181.html
Introductory Biochemistry Module: Energy Currency Molecules. http://www.prm.unisa.edu.au/h&p1ener.htm#atp