CASE STUDY: Smog Photochemistry Modeling


WARNING! This case study requires a solid chemistry background AND the willingness to tackle a large, complex system that requires solid planning PRIOR to building the model with STELLA®. This model also REALLY requires you to pay attention to units and conversion of units.
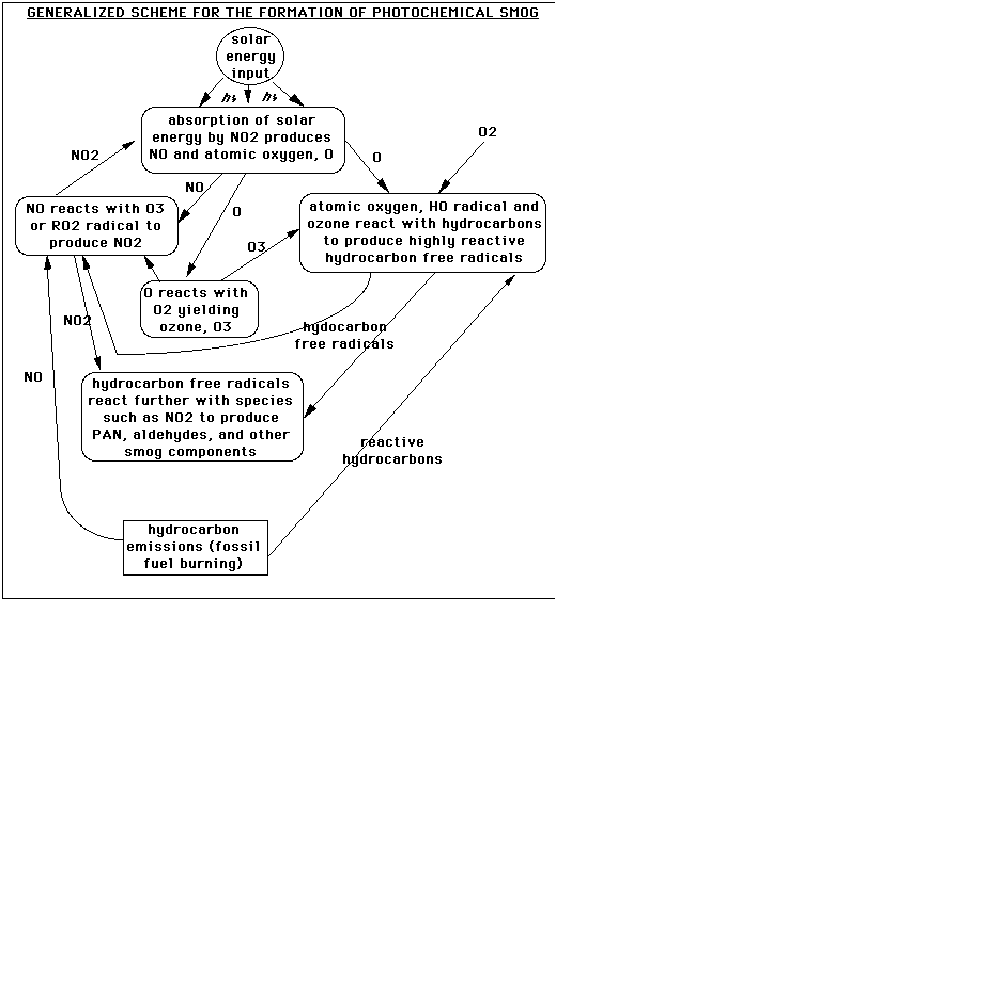
A schematic is again useful in understanding the chemistry of smog production in the troposphere:
This graphic is relatively self-explanatory. It begins at the bottom with the production of NO and reactive hydrocarbons by fossil fuel burning (such as an automobile). On the left side, the NO reacts with tropospheric ozone or a hydrocarbon radical (RO2•) to produce NO2 (a radical is a molecule fragment that has an unpaired electron). This absorbs solar energy (represented by the letters "hv") to create NO (which propagates the system) and atomic oxygen. Atomic oxygen reacts to form tropospheric ozone, which feeds back into the NOx system (the "x" here refers to the number of oxygens, and serves as a general notational term for the nitrogen oxides). Atomic oxygen can also react with hydroxyl radicals, OH•, and ozone to form the reactive hydrocarbon radicals utilized in the NOx system. These radicals also react to form other components of smog, such as PAN (peroxyacetyl nitrate) and aldehydes (RC=OH, where R is some hydrocarbon chain).
The graphic below shows the essential workings of the NOx system, with the interactions between NO and NO2 on the left and the production/washout of HNO3 on the right:

We can also look at the formation of photochemical smog from a kinetic perspective. This chart shows the nine key equations of smog production, and the rate constant that affects the speed (or rate) at which the reaction takes place:

In this graphic, we see the involvement of the NOx system and the production of ozone. Here is a narrative version of the graphic above:
In summary: the development of photochemical smog is dependent upon solar radiation, source emissions of hydrocarbons and nitrogen oxides, and atmospheric stability (for enhanced concentrations). Early in the morning, commuter traffic releases NO and hydrocarbons. At the same time, NO2 may decrease through because the sunlight can break it down to NO and O. The O is then free to react with O2 to form O3. Shortly thereafter, oxidized hydrocarbons react with NO to increase NO2 by midmorning. This reaction causes NO to decrease and O3 to build up, producing a midday peak in O3 and minimum in NO. As the smog ripens, visibility may be reduced due to light scattering by aerosols. Primarily due to the dependence on commuter traffic between suburbs and cities, there are presently more than 40 urban areas in violation of the US ambient air quality standard for ozone.
We can look at the change in concentrations of NO, NO2, and CO over time with this system of first-ordered differential equations. In the schematic below, we show the reactions by number from the graphic above. For example, R1 represents the photochemical reaction in which light energy hits a molecule of NO2 to form NO and singlet oxygen:
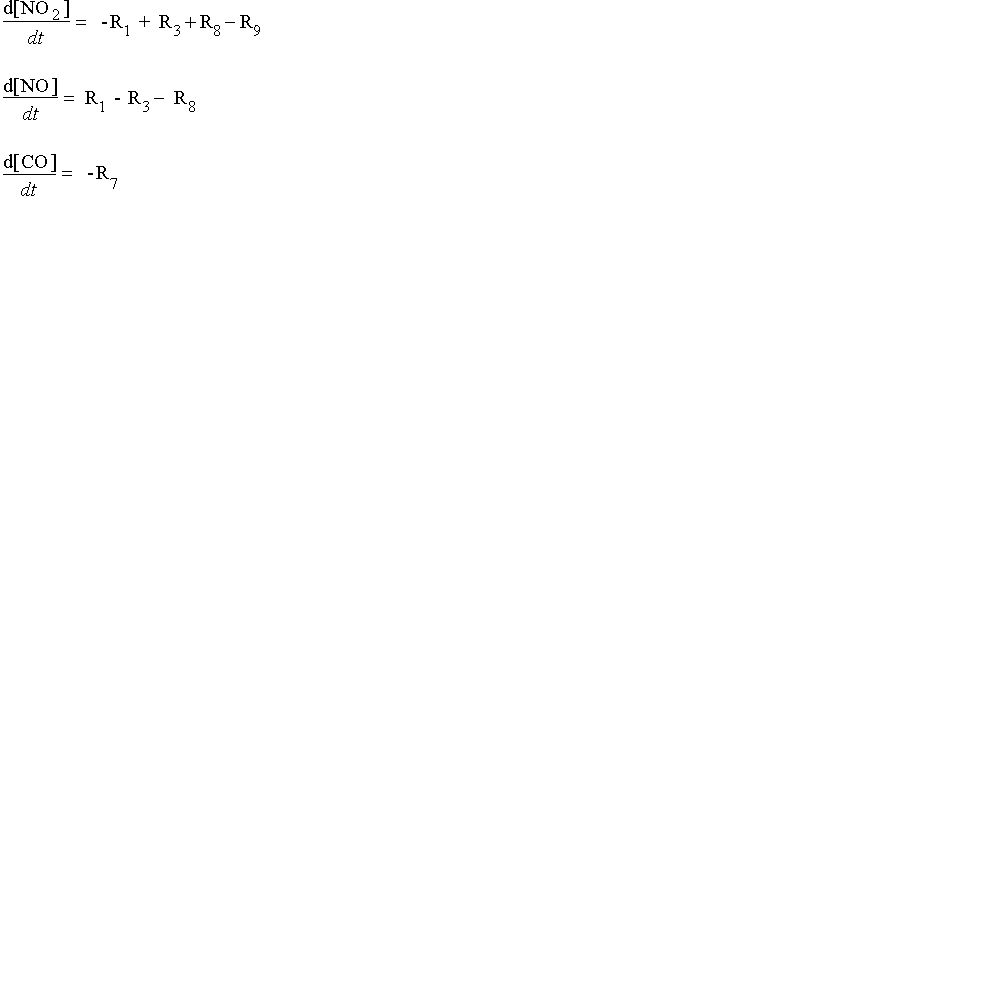
In the equations below, we substitute the kinetic components for each of the values of R:

In these equations, the term "k" refers to the rate constants, and are numbered following the reaction numbers in the graphic above.
| TRIAL | 1 | 2 | 3 | 4 |
| [NO2]o | 10 | 100 | 200 | 1000 |
| [NO]o | 10 | 500 | 500 | 500 |
| [CO]o | 50 | 10000 | 10000 | 10000 |
In each case assume that the photolysis rate constant for NO2 (R1 above) is:
In order for you to be able to build this model in a reasonable amount of time, I have done some of the algebraic rearrangements that need to be done to build this model in STELLA. You need to know that a "source" is an input, and a "sink" is an output.
Note that the reaction rates are in terms of molecules/cm3 not ppm, therefore your differential equations should work with molecules/cm3. Converters are used to translate molecule/cm3 to ppb (or ppm) which are more typical of air quality standards. Also many reaction rates are given as molecule/cm3-s, whereas the model is run in minutes.
**PAY CLOSE ATTENTION TO UNITS -- parts per billion, seconds, minutes, molecules/cm3, etc.
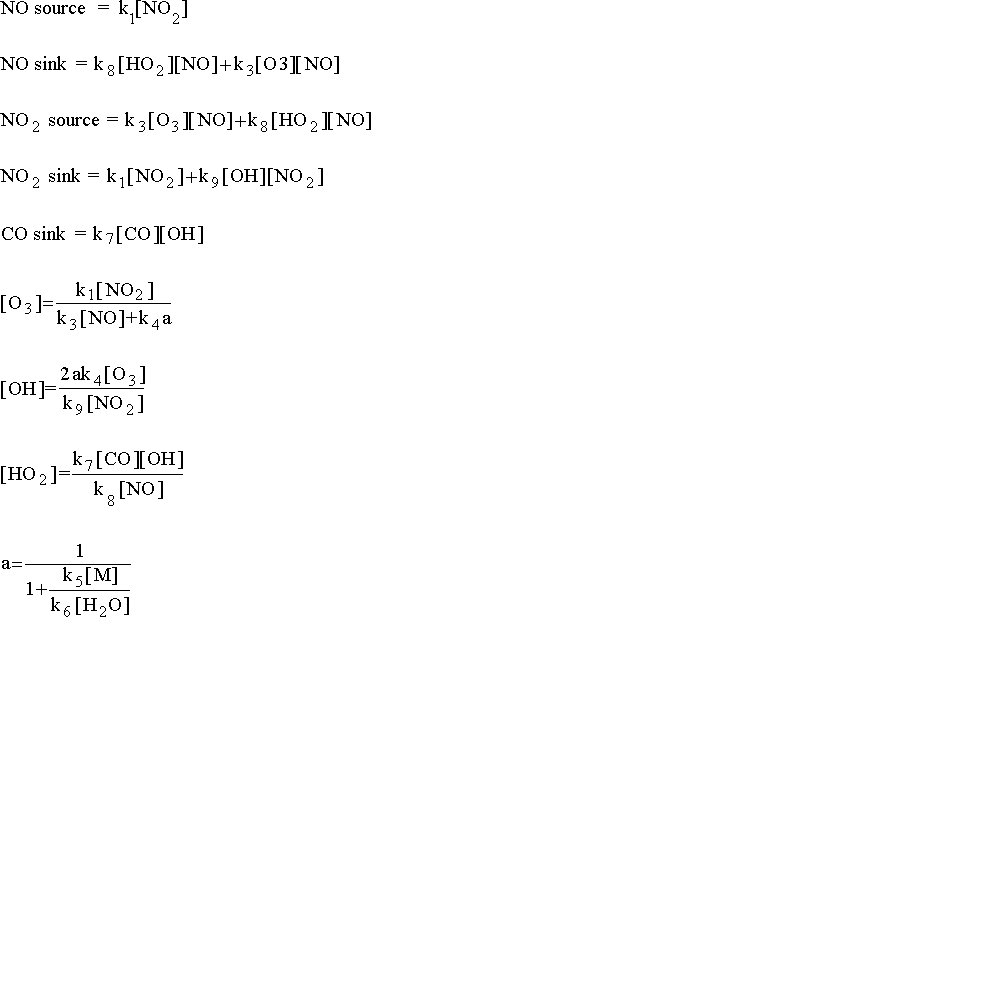
Once you have completed the model, experiment with various temperatures. You also might want to adjust the reaction rate of the first reaction, see what happens! You might also wish to run different starting values, such as are found in the Table of Inputs. If you have learned how, you might wish to consider doing multiple runs on one species.
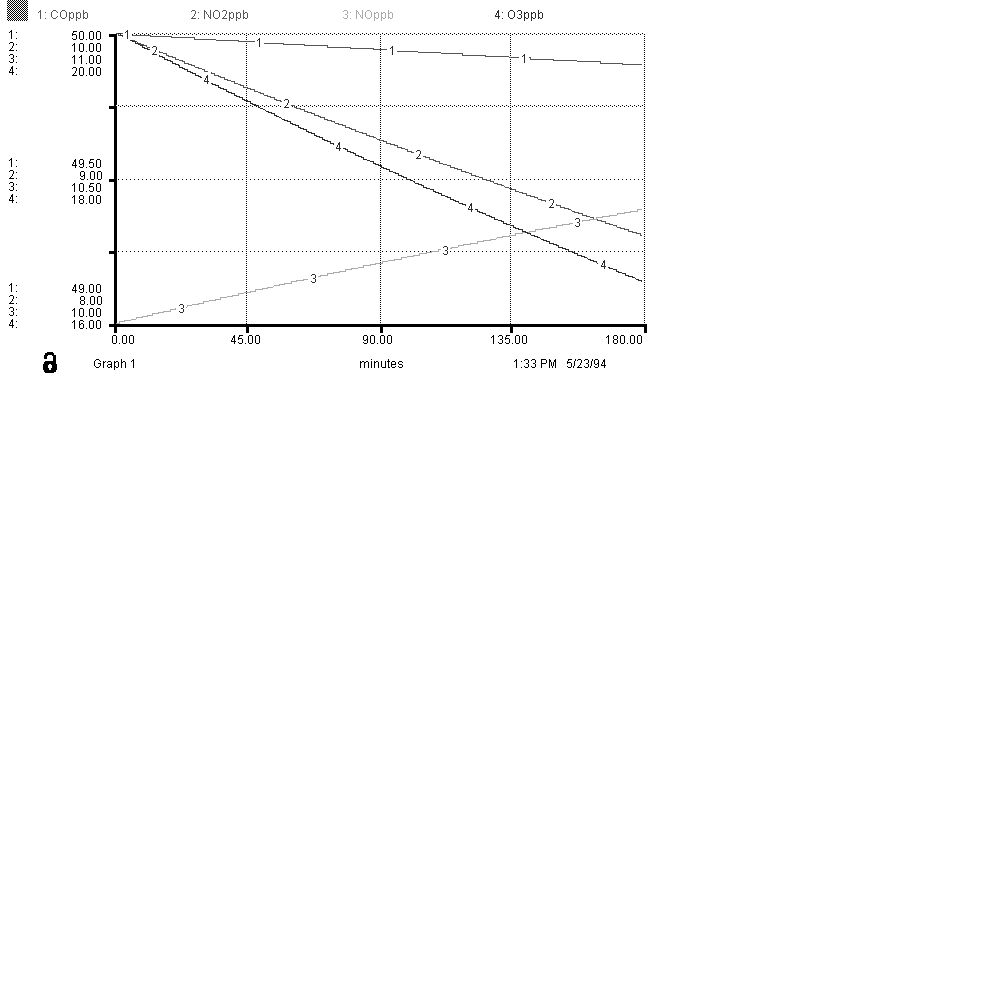
Below is a sample graph, in parts per billion, for the species of interest. Use this as a way to judge your model for accuracy and correctness.
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.