CASE STUDY: Transport of Atmospheric Pollutants


In this case study, we are concerned with the flow of a particular pollutant, ethane, between the Northern and Southern hemispheres. Ethane (C2H6) is a part of natural gas that is emitted to the atmosphere whenever natural gas escapes unburned at wells and other sources. Ethane only enters the atmosphere in this fashion. In the atmosphere, ethane undergoes a number of chemical transformations (mostly oxidation with the hydroxyl radical, OH•) to form peroxyacetyl nitrate, also known as PAN. PAN is a part of the smog and ozone problems, and causes severe eye irritations and respiratory problems, particularly among the elderly and those with respiratory diseases.
In the Northern Hemisphere, ethane has a concentration of about 1.0 parts per billion (ppb). With a concentration of 1 ppb, that means there is one molecule of ethane for a billion molecules of air molecules. Using a golf analogy, if the concentration of red golf balls was 1 ppb, there would be one red golf ball for every 1,000,000,000 white golf balls. This may not seem like a problem, but this one little molecule of ethane reacts chemically with other stuff in the atmosphere, and eventually becomes a problem. It is like rolling a small snowball off a long hill - a little snowball won't hurt anyone, but if it rolls long enough, it becomes a snow boulder that can do serious damage!
In the Southern Hemisphere, the typical concentration of ethane is 0.5 ppb. Ethane can exit from the atmosphere by any of three methods, or mechanisms:
Ethane can also flow between hemispheres. Ethane can flow from North to South and South to North. The graphic below shows all of the possible mechanisms:
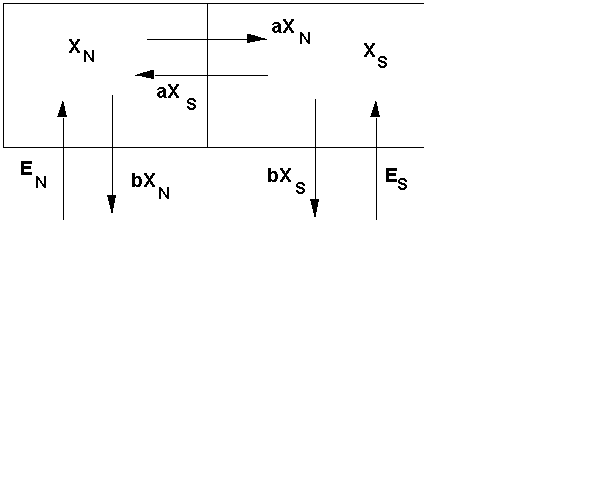
In this graphic, we show the Northern Hemisphere (XN) on the left and the Southern Hemisphere (XS) on the right. We show ethane coming into the two hemispheres from the bottom (EN and ES) . The graphic also shows ethane moving between hemispheres (aXN and aXN). In this case, the letter "a" is a rate that shows the number of ethane molecules transported per some period of time. For example, if "a" is 80%/year, then 80% of the ethane in the Northern Hemisphere and 80% of the ethane in the Southern Hemisphere change places every year. If the amounts in both are the same, then the net difference is zero -- this is called the steady-state or equilibrium condition. The politicians would love this to be true -- the stuff that is emitted in the North stays in the North, and the stuff emitted in the South stays in the South!
The graphic also shows ethane leaving both the North and the South through "wet deposition" -- pollutant mixes with the rain and snow and is deposited on the ground, where it may or may not do damage to people and things. Acid rain is the classic example of "wet deposition" - the stuff deposited is acid rain, which destroys buildings, plants, farm crops, and kills living things in ponds and lakes. The letter "b" is the rate constant for wet deposition from the atmosphere to the ground.
For different inputs (amount of ethane, EN and ES, emitted by the nasty polluters!), how long does it take (in years) until the stuff emitted in the North stays there and the stuff emitted in the South stays there -- in other words, how long does it take for a steady-state/equilibrium condition to be in effect?
Typical flows into the atmosphere of ethane are:
Run your model for a period of 50 years. Experiment with a suitable choice of dt, striving for the fastest run with the best accuracy. Similar, experiment with various choices for integration method.
Investigate a number of plots, such as plots of the concentrations of the two hemispheres. You should also establish a converter -- "net interhemispheric flow" that is the difference between the flow from North to South and the flow from South to North. Plot this interhemispheric flow with the concentrations of the two hemispheres. You might also investigate the flows themselves.
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.