The purpose of our project was to investigate the similarities of various molecular properties of artificial and natural sweetners to their corresponding relative sweetness values. Another major part of our project was to use the AH-B-X theory to find out the way in which sugars bond to receptor sites and why the shape of the molecule is important in defining a sugar. We researched relative sweetness values and solubility for aspartame, cyclamate, saccharin, and sucrose (table sugar); we calculated electrostatic potential, dipole, heat of formation, HOMO (Highest Occupied Molecular Orbital), and LUMO (Lowest Unoccupied Molecular Orbital) using MacSpartan.
For sweetness to be perceived by an individual, a molecule needs to have certain requirements. First, it must be soluble in the chemical environment of the receptor site on the tongue. It must also have a certain molecular shape that will allow it to bond to the protein in the taste bud. Lastly, the sugar must have the proper electronic distribution. This electronic distribution is often referred to as the AH, B system.
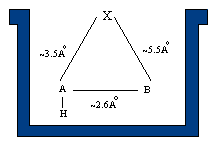
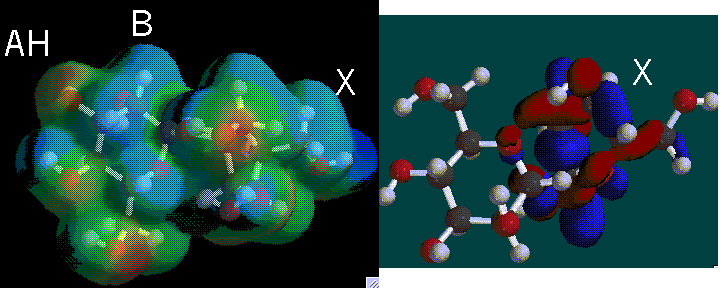
The present theory of sweetness, AH-B-X, suggests that there are three basic components to a sweetener. An AH, B, and X site are present and the three sites are often represented as a triangle.

The A and B regions of a molecule represent higher regions of electronegativity. Certain atoms represent these regions and the distance between them is greater than 2.4 A and less than 4.0 A. If the distance between the atoms are not in this range then molecule becomes bitter. The A part in the molecule becomes less electronegative because of the addition of a hydrogen atom that is bonded to the atom in the A region of the triangle and thus is referred to as AH and it becomes an electron acceptor. The second region, B, is an electron donor. The third part of this triangle is named X, which represents the hydrophobic and lipophilic region of the molecule. X is represented as a region, not as an atom, and does not bond to the receptor site.
AH and B regions are also present in receptor sites; the AH region of a sweetener hydrogen bonds to the B region of a receptor site, the B region of a sweetener hydrogen bonds to the AH region of the receptor site. The bonded protein and sugar is released and triggers a response by cells in the taste bud. The cells send electrical impulses to the brain creating the perception of sweetness.
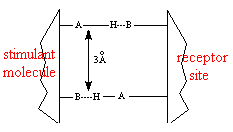
*Hydrogen bonds are represented by the dashed lines
The analysis we are doing is on three non-carbohydrate sweetners: aspartame, cyclamate, and saccharin. We are also looking at one carbohydrate sugar, sucrose, which is common table sugar. Aspartame is an artificial sweetener that is also known as "NutraSweet" and "Equal." It is very widely used as a low-calorie sugar substitute, being 180 times the sweetness of table sugar. There has been much controversy over the toxicity of aspartame, but it is still widely used throughout the world. Cyclamate is another low-calorie sweetener that is often used in combination with aspartame and saccharin. Although it has been banned in the United States, many countries take advantage of its sweetness, which is 30 times that of table sugar. Saccharin, the last of the non-carbohydrate sweeteners, is low-calorie as well and is 350 times sweeter than table sugar. It has been branded safe for human consumption and is the oldest artificial sweetener used today. Sucrose, or table sugar, was used as a basis to compare the three artificial sweeteners. Although there are many substitutes for sucrose, it is still widely used.
Solubility was also researched for the four molecules. The solubility of Aspartame is 10g per 100mL at 30 degrees Celsius when it has a pH of 2. Cyclamate is 20 degrees Celsius for 10g per 100mL. Saccharin is 24 degrees Celsius for 10g per 100mL. Sucrose is 19 degrees Celsius for 10g per 100mL. Solubility against relative sweetness is shown by the following graph.
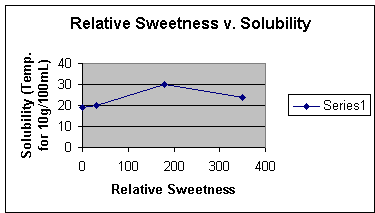
*The respective points are sucrose (1, 19), cyclamate (30, 20), aspartame (180, 30), and saccharin (350, 24)
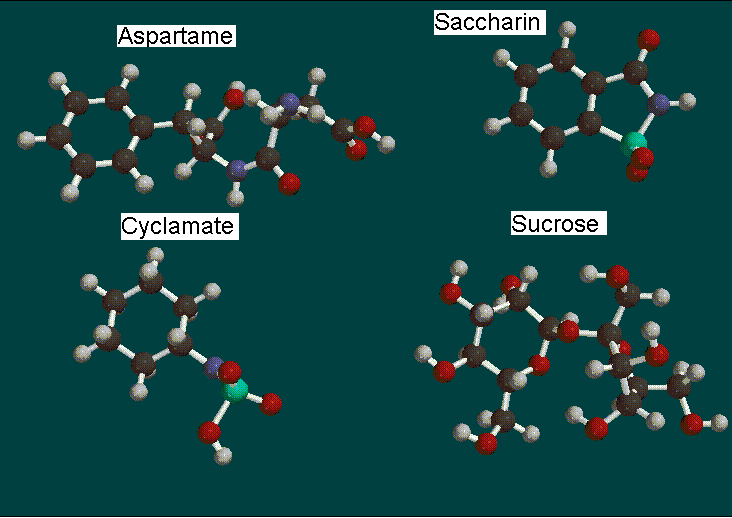
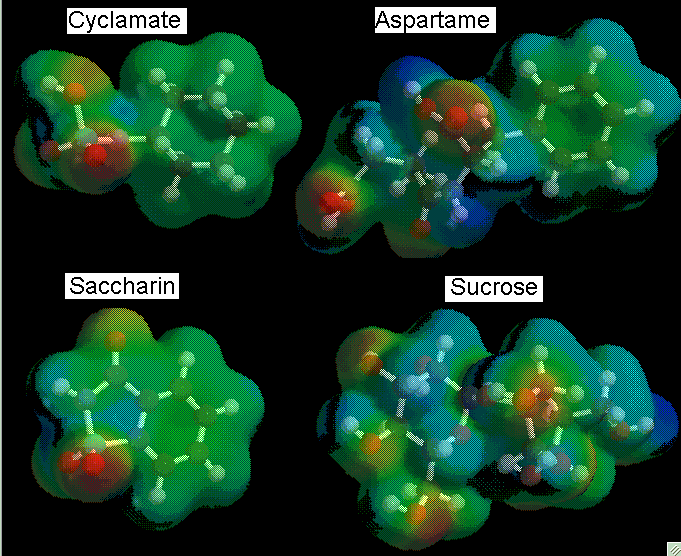
Mac Spartan, a molecule building program, running off of a power Macintosh performed all calculations and analyzations. We built each molecule according to their molecular diagrams.
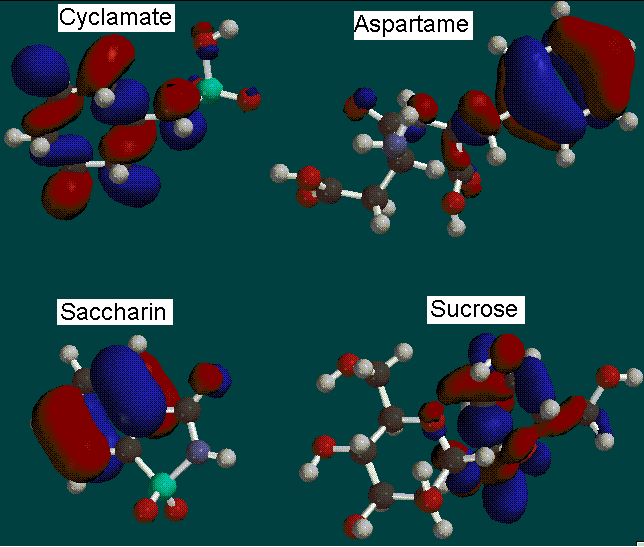
After each molecule was built and minimized, a geometry optimization calculation was performed using the AM1 basis set. The AM1 basis set was chosen to minimize calculation time and computer lock-ups. Our next calculation consisted of a surface density calculation with electrostatic potential (elpot) property. Then we proceeded to map these properties to view the electronic distributions of the molecule.
*Red indicates an electron-poor orbital, blue indicates electron-rich
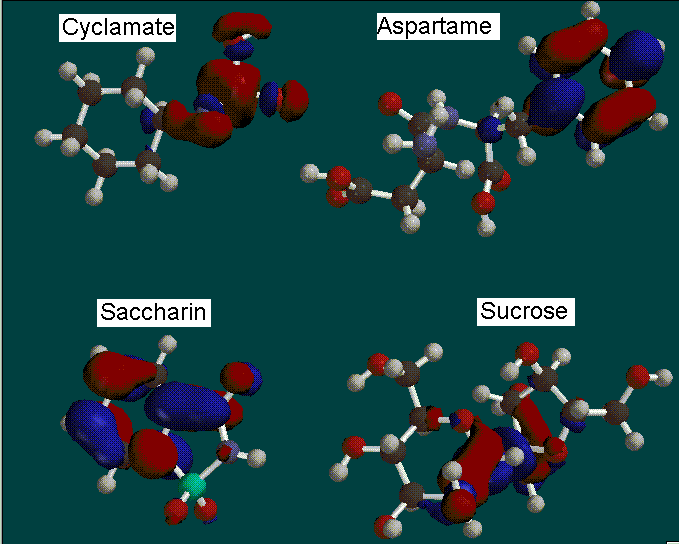
Other surface calculations included Highest Occupied Molecular Orbitals (HOMO)
*Red indicates an electron-poor HOMO, blue indicates electron-rich
and Lowest Unoccupied Molecular Orbitals (LUMO).
*Red indicates an electron-rich LUMO, blue indicates electron-poor
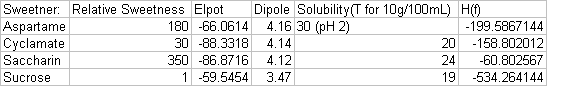
The computer then was asked to generate the dipoles and heat of formation for each molecule. After all calculations were performed and the data collected, we analyzed and compared each of the molecules for similarities. We assembled the data into a table.
We graphed the Relative Sweetness values versus the calculated data to form any relationships between these values.
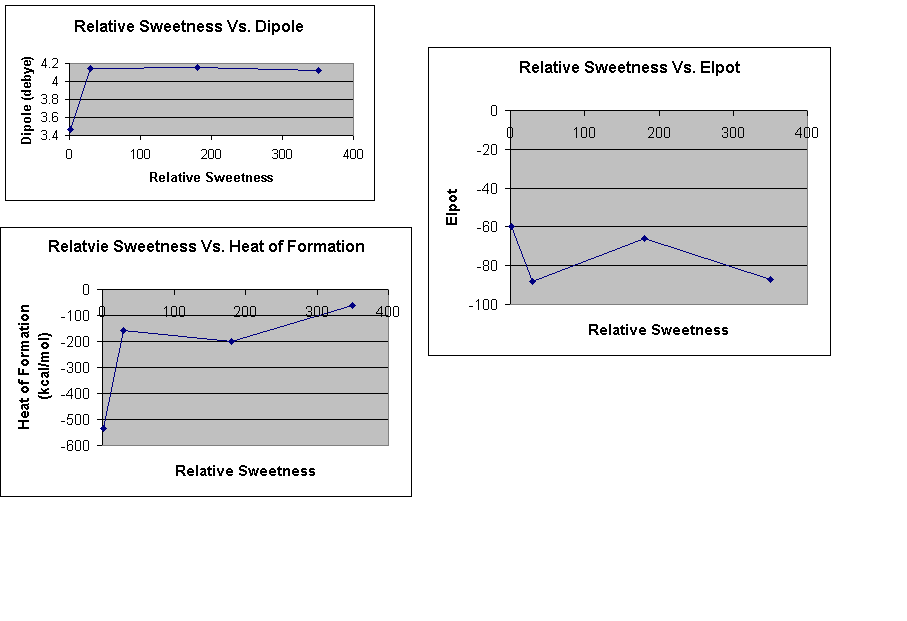
*The molecules are graphed in the following order: sucrose, cyclamate, aspartame, and saccharin.
We determined that the phenyl ring in the molecules are most likely characteristic of most sweeteners. In the three non-carbohydrate sweeteners, this phenyl ring is the X region of the molecule where it does not bond to the receptor site. In sucrose, the AH-B region occurs near the phenyl ring. For the HOMO graphs, the regions of blue and red are located around the X-region.
From the electronic distribution model for Cyclamate we determined that the left part of the molecule is the AH-B part of the molecule. The upper left where the Hydrogen is being the AH region and the lower part being the B region. The right side, where the molecule doesn't show signs of wanting to gain or lose electrons is the hydrophobic X-region. The region where more HOMO's are located is on the phenyl ring, which is the X-region of the molecule. We are not sure what this means but this looks to be a characterisitic of the X regions. There was no relationship found for the LUMO regions.
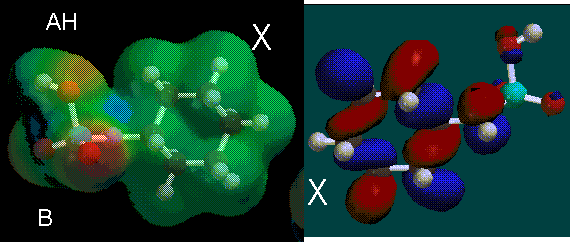
In aspartame, the AH region of the molecule is most likely located in the upper of the two electron deficient Hydrogen atoms between the two electron rich areas. This is because HOMOs are not present around this electron-poor region. The B region is most likely the electron rich area on the left where a HOMO is not present. The X region would be the phenyl ring where there is not much preference. More HOMOs are located on the X-region.
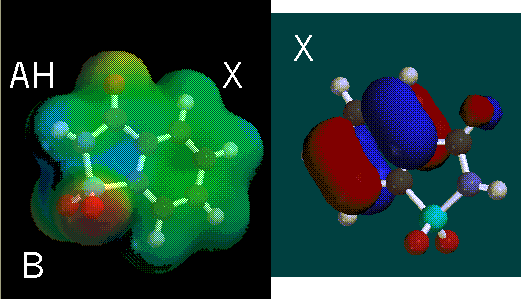
The AH region of saccharin is located in the middle left around the electron deficient hydrogen. The B region would be the more electron rich area of the molecule. More HOMO regions are shown on the X-region of the molecule.
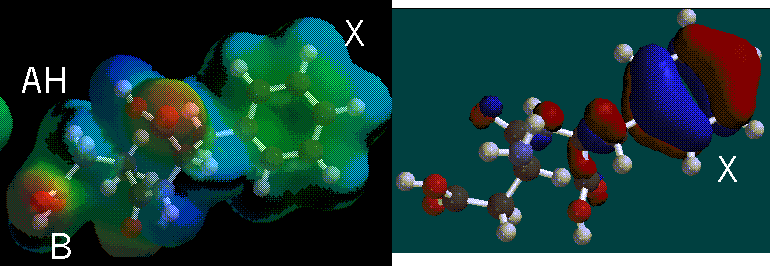
The AH and B regions of sucrose are little bit more obscure. By using a model from another researcher, the AH and B regions were found. The AH and B regions defined by a different model are supported by our model. The X region is located on the right side of the molecule and is supported by the model of the other researcher. More HOMOs are shown around the X-region.
The data from our project show that there is no direct correlation between sweetness and individual properties of the molecules. Putting together the properties of the molecules should reveal why a sugar has a certain relative sweetness. Sucrose is soluble at a lower temperature, has a lower dipole value, lower heat of formation value, and a lower electrostatic potential value indicating a lower sweetness. The other molecules didn't show much differences between themselves but all are greater than sucrose for the different properties. From other studies we looked to find a trend for electrostatic potential, dipole, and solubility, but found none.
When relative sweetness is compared with elpot, heat of formation, and dipole a relationship cannot be determined using the resources and data available. First, relative sweetness values are disputed; they were given as ranges. These ranges were then averaged to obtain the relative sweetness values. Next, the version of Mac Spartan used did not include the sodium ion. The molecular structure for cyclamate is really the structure for sodium cyclamate. Also, without including the sodium ion the elpot, heat of formation, and the dipole calculations are inaccurate because a Hydrogen atom was used in its place. This adjustment in the value, with including the sodium ion, could modify the data, resulting in the ability to determine a relationship.
By mapping the elpot and the HOMOs of each molecule we were able to determine possible AH, B, and X regions of the molecules and understand why these regions are reasonable predictions.
We would like to give special thanks to Bob2 and all the other Shodor staff.
Acree, Terry. Molecular Theory of Sweet Tastes. http://www.nysaes.cornell.edu/fst/faculty/acree/ahb/acree70.html
Dictionary of Sweeteners. www.daniscosugar.com/about_sugar/sweetlex/relativ.htm
Hamilton, Bruce. Sci. chem. FAQ. http://www.landfield.com/faqs/sci/chem-faq/part5/http://chemfinder.com
Immel, Stefan. Sucrose, Fructose, and Non-Carbohydrate Sweeteners: Correlations Between Hydrophobicity Potential Profiles and AH-B-X Assignments. http://caramel.oc.chemie.tu-darmstadt.de/~lemmi/Poster/INTNCARB_16/abstract.html
Marshall, Richard. Theory of Sweetness. http://www2.unl.ac.uk/~hx14marshar/sweetness.htm
Sweetness Chemistry. www.agsci.ubc.ca/courses/fnh/301/carb/carb4.htm