
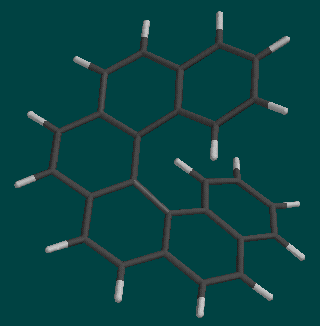
Helicenes are spiral molecules formed from fused benzene rings. A number in front of the "helicene" name indicates how many rings are involved. In this lab, we will be working with [5]helicene and [6]helicene, shown below.
 |  |
These spirals are unusual molecules because there are "right-" and "left-" handed forms. Helicenes are "chiral" because they have right and left isomers. The two forms can be differentiated by imagining that the spiral is the inclined plane of a screw. If you rotate a screw clockwise and it advances, it is right-handed. If you rotate it counterclockwise and it advances, it is left-handed. The conversion between right- and left-handed forms is called racemization, which you will investigate in this study.
In this study, you will construct a transition state for the racemization of [5] and [6]helicene. The procedure is the same for both molecules.
Construct a helicene in the builder and minimize it. It may help your construction to start with a benzene ring, and simply add aromatic carbons to it. Minimize often to make building easier. Make sure you know whether you are building a right or left handed spiral! (Know your chirality!) Optimize the geometry and save before you construct a transition state. (Hint: Unless you are using a high-powered workstation, ie, SGI workstation, you MUST use AM1 theory...you will run out of memory if you try to use ab initio methods).
Now construct a transition state for racemization. You can start with the previous geometry or begin again. The most obvious transition state, with all the rings in one plane, is not a feasible option; it may look possible for [5] helicene, but there is not enough space for [6] helicene to have a similar state. Rather, the most probable transition state is one where the rings alternate between an up and down tilt. Try to construct one like this, or a structure which you think is a possible transition state. Conduct a transition state search using the original helicene as the reactant and the possible state as the product. Make sure that you match up these spirals with the proper chirality. Confirm your transition geometry with a frequency search. What do you think the imaginary frequency should look like? How are the two transition states different and similar?
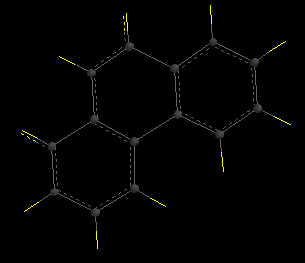
Hint for [5]helicene: build a planar structure with 5 benzene rings, with a methylene bridge across the gap (shown below). Minimize this structure with SYBYL theory, delete the methylene group, and use that as your transition structure.

Compare the geometry of the helicenes with the bond length and angles in phenanthene. How does the spiral structure change the geometry of the benzene rings?
Compare electrostatic potential maps of phenanthene and [5]helicene. How are they different? What does this indicate about the potential reactions that helicenes can undergo?
[Glossary][CompChem Main Page][HTML Labs]
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.