|
|
Movement of Sulfur CompoundsThe following graphic shows the movement of sulfur compounds through the troposphere and into the stratosphere. An important element to notice is the movement of OCS, carbonyl sulfide through the atmosphere. It is the most abundant sulfur species in the atmosphere due to its low reactivity: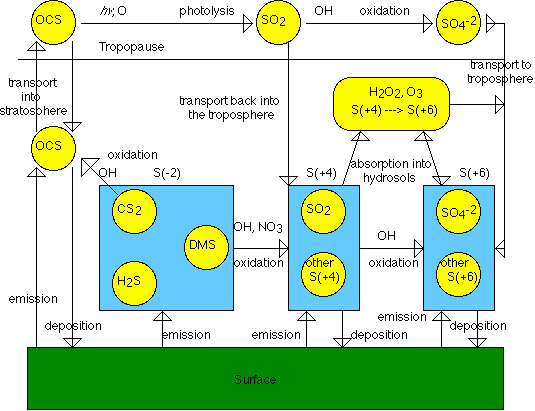 (image adapted from Seinfeld et al) Another important element to note in this graphic is DMS, dimethyl sulfide. DMS arises mainly from oceans and is produced by benthic and planktonic marine organisms. Its concentration is much higher in the oceans than it is in the atmosphere, and it is constantly in flux from the ocean to the atmosphere. The main reactions of DMS are with the hydroxyl radical (during the daytime) and the nitrate radical (during the nightime). The following model shows fluctuation in concentration from night to day.
Reaction with the OH radical:
followed by:
DMS can react with NO3 (which, as we know, drives the nightime reactions in the troposphere. . . right!): In the next section, we will discuss in depth SO2, which has the highest level of emissions of any of the sulfur compound! Confused? Have a question? If so, check out the Frequently Asked Questions (FAQ) page or send mail to the OS411 tutor (os411tutor@shodor.org) with your question! Report technical/content problems here |
|
|