What are CFCs?Experimental data show that chlorine and other halogens are a big part of ozone depletion. But there are only a couple of natural sources of the Cl radical and almost none of any other halogen. Where does it all come from? Humans produce some of the main sources of chlorine and bromine radicals through chemicals called chlorofluorocarbons and halons. CFCs contain carbon and halogens, especially fluorine and chlorine. Halons are like CFCs, but they contain bromine instead of or as well as chlorine. CFCs and halons are molecules that have strong bonds, which make them very stable. Chlorofluorocarbons are insoluble, nontoxic and have very low reactivity. For this reason, CFCs were considered very safe. CFCs could not be disposed through pollution in the troposphere or dissolved in rivers. In the 19070's , the amount of CFCs were measured. The amounts of CFCs were almost equal to the total amount of the chemicals ever manufactured! So what happened to the amount that had disappeared? CFCs were photolyzed by UV-rays with wavelengths between 185 and 210 nm. That reaction releases Cl and increases the rates of the ClOx cycles. Until that happens, CFCs remain in the stratosphere; the expected total lifetime of a CFC (in the atmosphere and during the ClOx cycles before being removed permanently) can be anywhere from 60 to 400 years depending of the CFC. 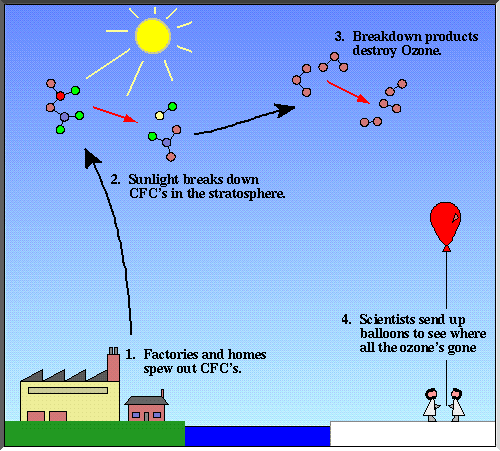 Report technical/content problems here |