| |
Particle Size
 |
Particle size generally refers to the diameter of a particle.
Both particle volume and and area are used in some applications.
The particle diameters of interests in studying atmospheric aerosol
behavior range from molecular clusters of about 10-3 mm
to cloud droples and dust particles as large as 100 mm.
In air pollution applications, particles larger than 1 mm
are called coarse mode.
Particles that are smaller than 1 micron (submicron particles) can be
divided into the accumulation mode,
from 0.1 to 2.0 mm, and the ultrafine
mode consisting of particles that are smaller than 0.1 mm.
There is such a wide range in the particle sizes of interest in the atmosphere,
(varying in size by as much as105 and in mass by
as much as 1015 ), that some specialized graphical and mathematical
tools have been developed in order to represent the whole size distribution
of interest in atmospheric studies. The most generally useful of these
representations will be discussed later in this unit.
The size distribution of atmospheric aerosol results from dynamic processes
that affect both natural and manmade aerosol. There are usually two
modes in the mass distribution: the coarse mode corresponding to particles
larger than 2 to 3 mm in diameter and the accumulation
mode between about 0.1 and 2.5 mm. Distribution
like this have been repeatedly observed in many different locations.
For liquid or spherical particles, the particle size is easily
characterized by diameter. However, atmospheric aerosol are frequently
non-spherical. There are several application specific ways of determining
"equivalent" particle diameters but the most commonly is the aerodynamic
diameter. Aerodynamic diameter is the diameter of
a sphere with a density of 1 that has the same terminal settling velicity
as the irregularly shaped particle being measured.
|
Terminal settling velocity is determined using the formula:
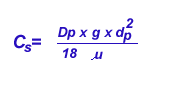
where Dp is the particle density, g is the gravitational acceleration
( 980 cm/sec2) , dp is the particle diameter, m
is the viscosity of air.
The animation below shows a spherical
particle with a density of 1 (blue) and an irregular particle with the
same density, and therefore the same settling velocitiy (green).
The aerodynamic diameter of the green and blue particles are the same.
The red particle also has a density of 1 but it falls at a much slower rate. Therefore it must have a
smaller aerodynamic diameter than the other two particles.
Three particles of the same density but one has a different aerodynamic diameter
The optical properties of aerosol are closely related to particle size
because interaction of small particles with light is a sensitive function
of particle size and optical properties of the airbonre particles.
Light scattering, a propertiy of interest in air pollution science because
of its affect on visibility, depends on the ratio of diameter to the wavelength
of incident light,
dp/ l,
where dp is the particle diameter,
and l the wavelength
of the incident lightlight.
Atmospheric chemical and physical properties lead to the accumulation
of particles in the size range of that produces the most severe effects
on visibility. The particle size range that scatters the maximum
amount of light per unit mass of material is near
0.2 mm depending somewhat upon other optical properties of the particle.
Confused? Have a question? If so, check out the Frequently Asked Questions (FAQ) page or send mail to the OS411 tutor (os411tutor@shodor.org) with your question!
Report technical/content problems here
|