NOx ChemistryNitrogen-containing compounds are key players in any discussion of tropospheric chemistry. Typical nitrogen containing compounds include nitrous oxide (N2O), nitric oxide (NO), and nitrogen dioxide (NO2). NO and NO2 are collectively known as "NOx", where the x represents a subscript. The principle source of NOx is the high-temperature combustion of fossil fuels. There are two primary sources of NOx formation:
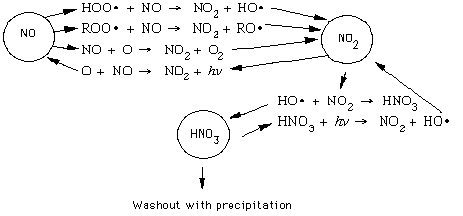
In the thermal process, high temperature and high oxygen concentration favor NO formation. Rate constants for thermal NOx production in the forward reactions are temperature dependent, while only the oxidation of NO is temperature dependent. The mechanisms for fuel-based NOx formation are not fully understood. It is known that the amout of NO formed during this process depends on the fuel-to-air equivalence ratio. The amount of fuel NOx formed is not temperature dependent, but rather is a linear function of nitrogen content, usually measured in percentage (by weight). There appears to be little difference between fuels that are derived from petroleum, shale, or coal. NOx sources are shown below.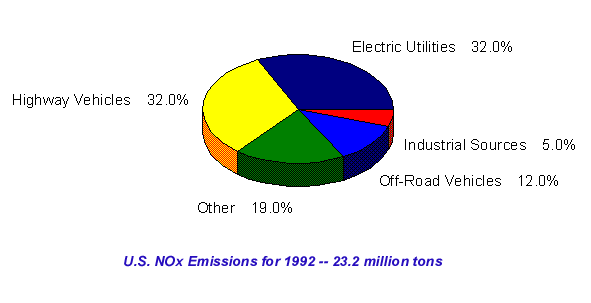 . .Important NOx are shown belowfor the three key nitrogen-containing compounds found in the troposphere: nitric oxide, nitrogen dioxide, and nitric acid (HNO3). With NO, reacts with free radicals to form NO2. It can also be oxidized in a reaction with atomic oxygen to form NO2 and O2. NO is also produced by the photolysis of NO2 to form NO and a singlet oxygen. NO2 reacts with a hydroxyl radical to form HNO3, that is subsequently washed out of the troposphere by rain. It can also produced by the photolysis of nitric acid, that results in the formation of a hydroxyl radical.
 . .Report technical/content problems here |
|
|