The Nitrogen CycleNitrogen cycles through the atmosphere in an analogous manner to carbon. One model for the nitrogen cycle is shown below. The transfer rates and reservoir sizes are in the same units as with the carbon cycle. The atmosphere contains a large amount of diatomic nitrogen gas (N2), which is largely inert. The two main processes in the nitrogen cycle are that of fixation (the combination of nitrogen with other compounds) and de- nitrification, or the removal of nitrogen. These two processes work to maintain a steady state concentration of N2 with a turnover rate of about 1 X 107 years. Various physical processes, such as weathering, leaching, volcanic activity and crustal degassing, assist in transforming nitrogen compounds into atmospheric constituents. Nitrogen fixation can occur by a of methods, including electrochemical, photochemical, and biological mechanisms. Microbial decomposition also plays a role in the larger cycle. Without organic de- nitrification, all the world's nitrogen would be maintained in reservoirs in the oceans and in organic sediments. Each of the various processes that contributes to the overall nitrogen cycle are in and of themselves topics for considerable study and analysis. Again, this is an area that is made more accessible through the use of computational modeling tools and techniques. 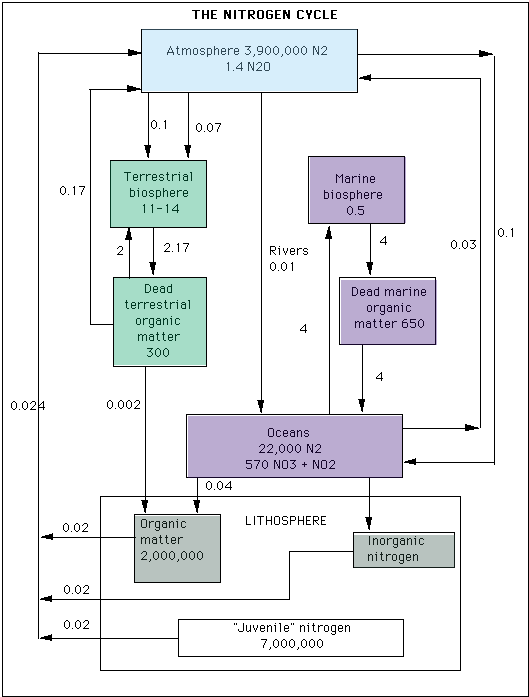 (Source: adapted from Wayne, R.P. Chemistry of Atmospheres, Clarendon Press, Oxford, 1991) Report technical/content problems here |
|
|