ConvectionHave you ever noticed that before water in a pot starts to boil, it seems to move and roll around in the pot? Just as pressure differences can drive motion, so can temperature differences. The ideal gas law gives us an idea of what happens when we heat a gas. As PV=nRT, for a process with little change in volume, an increase in temperature leads to an increase in pressure. This will create a pressure difference, which drives motion. In the case of convection, the standard model is that of a fluid being heated from below. The heated fluid undergoes an increase in pressure. Due to a pressure difference, the fluid will be forced upwards. As the fluid moves upwards, it will displace the cooler fluid above. At the location of the heating of the fluid, as the heated fluid moves away, this will draw nearby material in, allowing the cooler fluid from above which has been displaced to drop. 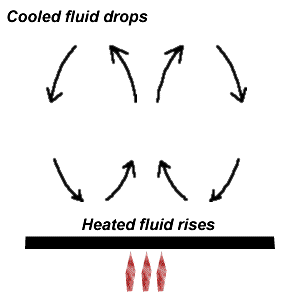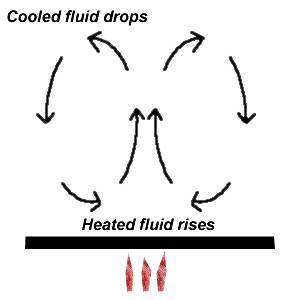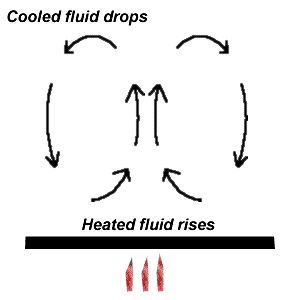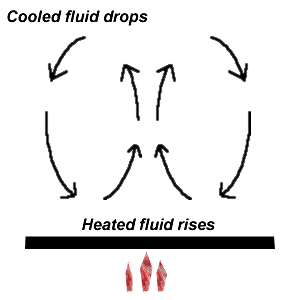 Report technical/content problems here |