Heat and Work
Heat and work are related forms of energy. One can be transformed
into the other. Whenever energy is transferred from one object to another,
it appears as work and/or heat. Work is the energy transferred when an
object is moved by a force, like the inflation of a football. The gas inside
the football exerts a force on the walls which in turn exerts a force on
the air surrounding the football. Heat energy (such as is used by a steam
engine) can be made to do work, for example, pushing a train down a track.
Work can also be transformed into heat. Consider the heat that is generated
by rubbing your hands together on a cold day.
Work and heat can both be described using the same unit of measure. The calorie is the unit of measure in most common use, and is defined as the amount of heat required to raise one gram of water by one degree Celsius. heat energy is generally measured in kilocalories, or 1000 calories. The standard SI units are Joules (J) and kilojoules (kJ). One calorie of heat is equivalent to 4.187 J. You will also encounter the term specific heat, the heat required to raise one gram of a matter through one degree Celsius. Specific heat, "C", can be calculated from the mass of material and the change in temperature as follows: =C = q / M D T where C is the specific heat, q is the heat added in calories, M is the weight of the material in grams, and DT is the rise in temperature of the material in degrees Celsius. The value of C for water is 1.00 calories/gram-degree Celsius. The values for specific heat that are reported in the literature are usually listed at a precise pressure and/or volume, so you need to pay attention to these settings when using values from textbooks, in problems, or in computer models. The amount of heat or energy required to bring about changes
in the state of matter are given special names. The heat of fusion is the amount of heat required to melt a substance. If the substance is
found in liquid form, the same quantity of heat is must be removed from
the matter to "fuse" it, or convert the liquid form to its solid form.
The heat of vaporization is the heat required to evaporate
the substance at its normal boiling point. Again, if the substance is in
its vapor form, the same amount of heat must be removed from the substance
to convert it from a gas to a liquid. While the magnitude of the heat is the same, the sign is opposite. Heat added is a positive q; heat removed is a negative q.
Chemical reaction frequently involve the transfer of energy in the form of heat from a material with a low specific heat to a material with a high specific heat where the energy is more effectively stored. Water has a very high specific heat and is valued as a good insulator for that reason. Metals are good conductors --- they have low specific heat and so effectively transfer heat. This runnable model demonstrates the transfer of heat from a metal to water.. Here is a sample problem that shows the calculation of work energy from information contained in a balanced chemical equation for a combustion reaction. 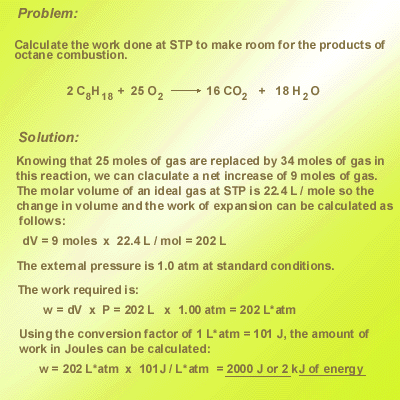 Report technical/Content problems here |
|
|