| |
Units and Unit Conversions
All measurements include both a number and
the units associated with that measurement. Ratios have ratios of
units in place of simple units, for example, miles/hour. The
most widely accepted unit system employed in scientific measurements is
the SI System of Units, from
the French, Systeme International d'
Unities. The SI system uses a set of 7 independent base
units from which all other units are derived by simple algebraic operations.
Some derived units that are particularly useful have unique names, for
example, volume is the special name for the units derived from cubing
meters (m), or m3. To identify particularly large
or small numbers of units scientific notation can be used but it is more
common to use decimal prefixes with SI units.
| DIMENSIONS |
UNIT NAME |
ABBREVIATION |
EXAMPLE OF DERIVED UNIT (Special Name) |
| mass |
kilogram |
kg |
density : kg m-3 |
| length |
meter |
m |
force: m kg s-2
(Newton, N) |
| time |
second |
s |
electrical charge : s A
(Coulomb) |
| temperature |
kelvin |
K |
(oC) |
| electric current |
ampere |
A |
potential difference : m2kg s-3 A-1 (Volt) |
| amount of substance |
mole |
mol |
concentration : mol m-3 |
| luminous intensity |
candela |
cd |
luminance : cd m-2 |
|
STANDARD PREFIXES USED WITH SI UNITS
FACTOR |
PREFIX |
SYMBOL |
| 10-12 |
pico |
p |
| 10-9 |
nano |
n |
| 10-6 |
micro |
m |
| 10-3 |
milli |
m |
| 10-2 |
centi |
c |
| 10-1 |
deci |
d |
| 101 |
deca |
da |
| 102 |
hecto |
h |
| 103 |
kilo |
k |
| 106 |
mega |
M |
| 109 |
giga |
G |
|
Unit Conversions
There are Many techniques
for performing unit conversions, however, one of the most widely used methods
relies on the creation of conversion factors. Equivalent quantities are expressed as ratios to
construct a conversion
factor. An everyday example of a conversion faction would be 60 minutes/1 hour. In the following example, multiplying a quantity by a conversion
factor changes the units in which it is expressed, but it does not change
the quantity. Choose the conversion factor that cancels all units
except those required for the answer. Set up the calculation so that
the unit of the quantity you are converting from (the beginning unit) is
on the opposite side of the division line (numerator or denominator)
from the unit of the quantity you are converting to (final unit).

Comparing Volumetric and Gravimetric Unit
There are two sets of units most commonly used to describe air pollution concentration -- volumetric
and gravimetric units. The units are used to identify the amount
of a particular pollutant gas either by volume or by mass, that might be
extracted from a unit or air. Volumetric units give the mixing
ratio between the pollutant gas of interest and the original volume of
air. Owing to fact that a fixed volume of any gas at a given pressure and
temperature always contains the same number of gas molecules, regardless
of the identity of the gas, this ratio also corresponds to the ratio of
the number of pollutant gas molecules to the number of air molecules in
the original gas volume.
This concentration can be expressed as parts of pollutant
gas per million parts of air, or ppm (also ppmv to indicate a volume rather
than a mass ratio.) Concentration is also shown mmol/mol
to comply directly with the SI base unit for amount of substance, the mole, (where a mole of any substance is defined 6.022 x
1023 molecules of that substance).
|
Conversion between gravimetric and volumetric units relies
on the application of the combined gas law,
PV = nRT
where P= pressure, V= volume, n= the amount of substance in moles
and R is the universal gas constant. At standard temperature and
pressure, STP,
(25o C, 760 mm Hg) 1 mole of any gas or mixture of gases
occupies 0.0224 m-3, and 1 mole of a pure gas weighs
M
kg where M is the molar mass (molecular weight) of the gas.
Abbreviations for Volumetric and Gravimetric Units
| Volumetric Units |
Gravimetric Units |
| ppm |
10-6 |
ul l-1 |
mmol mol-1 |
mg m-3 |
| ppb |
10-9 |
nl l-1 |
nmol mol-1 |
mg m-3 |
| ppt |
10-12 |
pl l-1 |
pmol mol-1 |
ng m-3 |
|

|
It
is uncommon, although sometimes possible, to see volumetric concentration
expressed as ml / l (microliters per liter).
Most data reports,(and consequently most models), still require input
in ppb, or for very low concentrations of ppt (parts per trillion)
as the units for volume-based concentration. Volumetric units of concentration
are unaffected by changes in temperature and pressure. As a result
they are especially useful where large fluctuations in temperature and
pressure during sampling are difficult to measure.
Gravimetric units delineate
the mass ratio of a pollutant to a specific volume of air. The units
are mg m-3. Unlike volumetric
units, gravimetric units can be used for solid or liquid particles as well
as gaseous pollutants. They are generally used when the sample is
collected on a filter and so the concentration relates directly to the
mass of contaminant collected for a measured volume of air sampled.
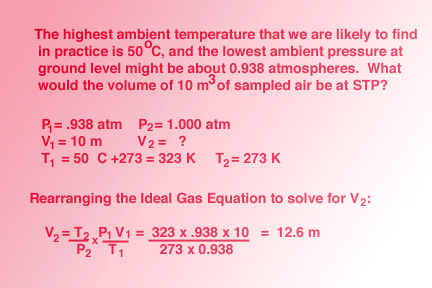
Standard Temperature and Pressure Corrections
For most air
quality measurements, temperature and pressure are unlikely to be at standard
conditions. It will be necessary to correct gas volume measurements
to correct to standard conditions in order to compare the gas sample collected
to other samples that have also been collected at a variety of non-standard
conditions. The
Ideal Gas Equation is used to perform these corrections.

|
 |
Report technical/Content problems here
|
|
