| |
The Ionic Bond
 |
Ionic
compounds
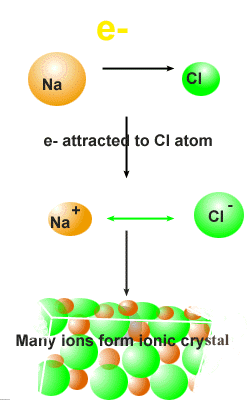
are made up of ions or charged
particles that form when an atom, or a small groups of atoms, gains or
loses one or more electrons. Ionic compounds generally
form when a metal reacts with a nonmetal. Each metal
atom loses a certain number of its electrons and becomes a cation
while the nonmetal gains the electrons lost by the metal atom and becomes
and anion. When the opposite charges of
cation
and an
anion are strongly attracted to each other , an
ionic compound results. The resulting compound is electronically
neutral. All ionic compounds are crystalline solids with an array
of cations and anions stacked together. The faces of the crystal are where
the stacks of ions come to an end.
Click on ionic bonding video clip to see an animated visualization of this process. |
You can predict the relative charge on a monatomic ion from the
position of the parent main
group elements in periodic table Transition
metals often form cations with more than one relative charge and are more
difficult to predict. Main group elements generally lose or gain
electrons to form ions with the same number of electrons as the nearest
noble gas ( Group 18 elements), so that all halogens form -1 ions while
group 16 elements like oxygen and sulfur form -2 ions.

| When a small group of elements bind together but form a charged
species rather than a neutral compound, it is called a
polyatomic ions. The bonds within the polyatomic ion are covalent
but the combination of atoms does not result in a neutral compound but
rather an ion is formed. The sulfate, SO42- carbonate,
CO32- and nitrate, NO3 - ions
are environmentally important examples of polyatomic ions. Polyatomic
ions can also be involved in ionic bonds to form ionic compounds like sodium
sulfate or calcium nitrate. |
 |
Report technical/Content problems here
|
|