| |
The Periodic Table
| There are 90 naturally occurring elements and about 26 additional elements
that do not occur naturally on earth. The number of elements is periodically
revised based on data obtained from experiments in particle accelerators,
large research facilities that simulate some of the extreme conditions
that are thought to have existed when our universe was formed. For example,
scientists at Lawrence Berkeley Laboratories
recently detected elements 116 and 118 for the first time. The regular
arrangement of elements in the periodic table of the elements reflects
patterns in structure and behavior observed experimentally. In fact,
there are still some aspects of the regular patterns of behavior among
the elements that cannot be explained by current theories. |
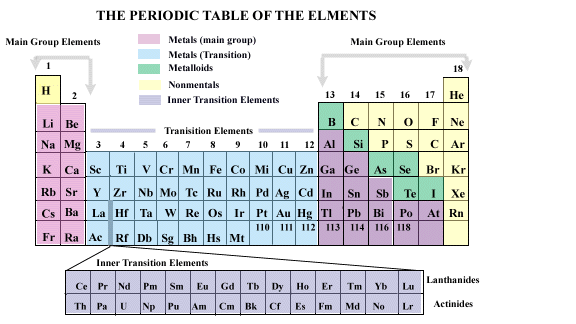 |
Elements in the periodic table are arranged horizontally in rows or
periods
Each successive element is larger by one proton than the element to its
left leading to a number of properties that change in regular and predictable
ways as you move across a period. Groups
of elements with very similar chemical properties are arranged vertically
in the table. Elements arranged in vertical groups in the periodic table
have the same number of valence electrons,
that is the electrons in the atom of an element that are least attracted
to, and are farthest away from the atom's nucleus.
You can explore these and many more features of the periodic table by trying out the interactive periodic table provided in the Tools pull down menu above, or by visiting the WebElements website.
|
Report technical/Content problems here
|
|